The following represents a suggested workflow/steps for closing out your study (i.e. getting to lock) and archiving it. Note that the statuses of your Production and Test environments for each study are controlled independently.
Sign records
- The Investigator user role at the site level has the ability to sign records. The study level user role called “Data Specialist,” can also sign records (if you choose to use it). (more about signing)
- Events must have a status of completed before they can be signed.
- Users who can can sign may do so at the event or casebook level:
- Casebook signing. The user can sign a casebook if all scheduled events for that participant have a status of “completed”, “stopped”, or “skipped”.
- Event signing. If all the required forms for a particular participant’s event have a status of complete and no non-required forms in the event are still in-progress, then the user can sign the event by going to the edit screen for that event and choosing Sign. Note: The user will need to mark the event “complete” first on the edit screen in some cases when the system does not do it automatically.
- Tip: Use OpenClinica Insight reports to show what data has been signed / not signed and Pulses to automatically send this information to investigators throughout the course of the study.
Freeze the study. Freezing is a status that prevents data entry or any data changes, but allows queries to continue. Data managers can freeze individual sites or the entire study environment by editing the site or study settings, respectively. Data may be unfrozen in this same manner.
- The Participant Matrix will provide a clear indication of which data has, or has not, been locked. This can be supplemented with more detailed Insight reports.
- If it is important for the data to be signed before being locked in your study, you will need to verify this on your own as the system will not require the data to be signed before being locked.
- It is simple to unlock an event, site, or study in the same way you lock it (by editing the settings for that object).
Archive Data. Once the study environment is locked, we recommend archiving your data by extracting it and printing casebooks. Note: OpenClinica will retain your data for as long as you have a subscription. However, if your study environment is in Archived status, you will not be able to access the study data without reactivating it. So, this step may be optional, or could be performed at a later time.
- Extract your study data in the CDISC ODM XML Full with OpenClinica Extensions format. This extract format includes all your study metadata as well as query related data and audit log data.
- Print casebooks from the Participant Details page. You can print archival casebooks, including queries and audit trail information, giving you a complete record of the participant in the study.
You can print PDF casebooks one participant at a time, or print them all patients in the study (or at a site) by using the JSON or XML format. Select the format (JSON or XML), then click Get Link to view the modifiable URL.
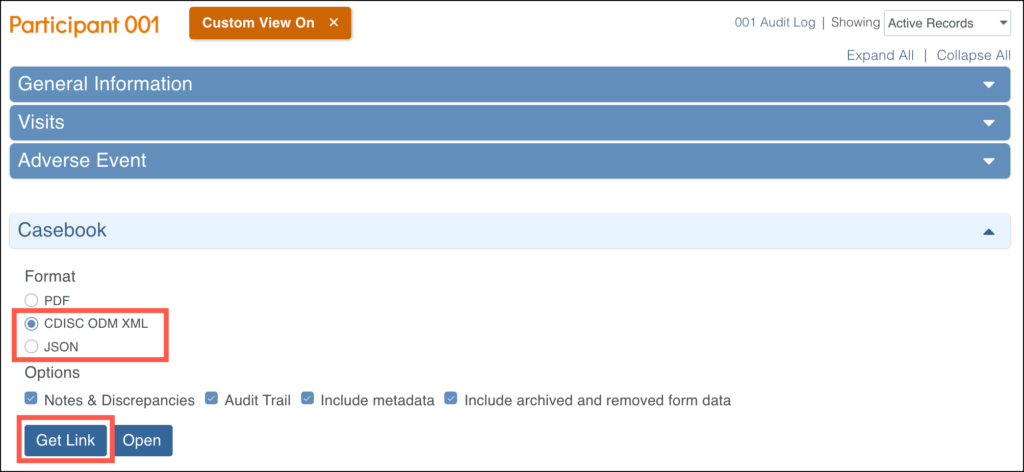
For example, the following URL would show the casebook for one participant: https://acme.openclinica.io/OpenClinica/rest/clinicaldata/xml/view/S_DF/SS_DF001/*/*?includeDNs=y&includeAudits=y&includeMetadata=n&showArchived=y
Replacing the SS_DF001 with an asterisk (*) will give you casebooks for all the subjects for this site in a single file. (https://acme.openclinica.io/OpenClinica/rest/clinicaldata/xml/view/S_DF/*/*/*?includeDNs=y&includeAudits=y&includeMetadata=n&showArchived=y)
Change the Production study environment status to ‘Archived’. This will remove the study from counting against your subscription limit. You may reactivate the study environment at any time, by updating the status. Please note that this does not have any impact on the study in OpenClinica Insight. If you would like to remove the study from OpenClinica Insight, please deactivate the Insight module on the Module Management screen prior to archiving your study environment. This will ensure that Insight is disconnected promptly, and you do not accumulate any unexpected Insight fees.
If you archive both the Production and Test environments for a study, it will move to the Retired studies page within My Studies instead of the default Active studies page.
