Part 11 Compliance for Electronic Signatures: Please note that in order to comply with Part 11, an electronic signature must include the participant’s first and last name. Because of this requirement, for your eConsent signature to be fully compliant, your study must be designed to require first and last names to be collected prior to the signing of the eConsent form. You could do this either by including the first and last name contact fields in the informed consent form itself and only showing the eConsent form after they’ve been filled in, or by creating a different contact form that must be filled out by a CRC or the participant (if also using Participate) ahead of time. See Design Participate Forms for how to configure forms to capture first and last name in a secure manner for use in participant eConsent signatures. |
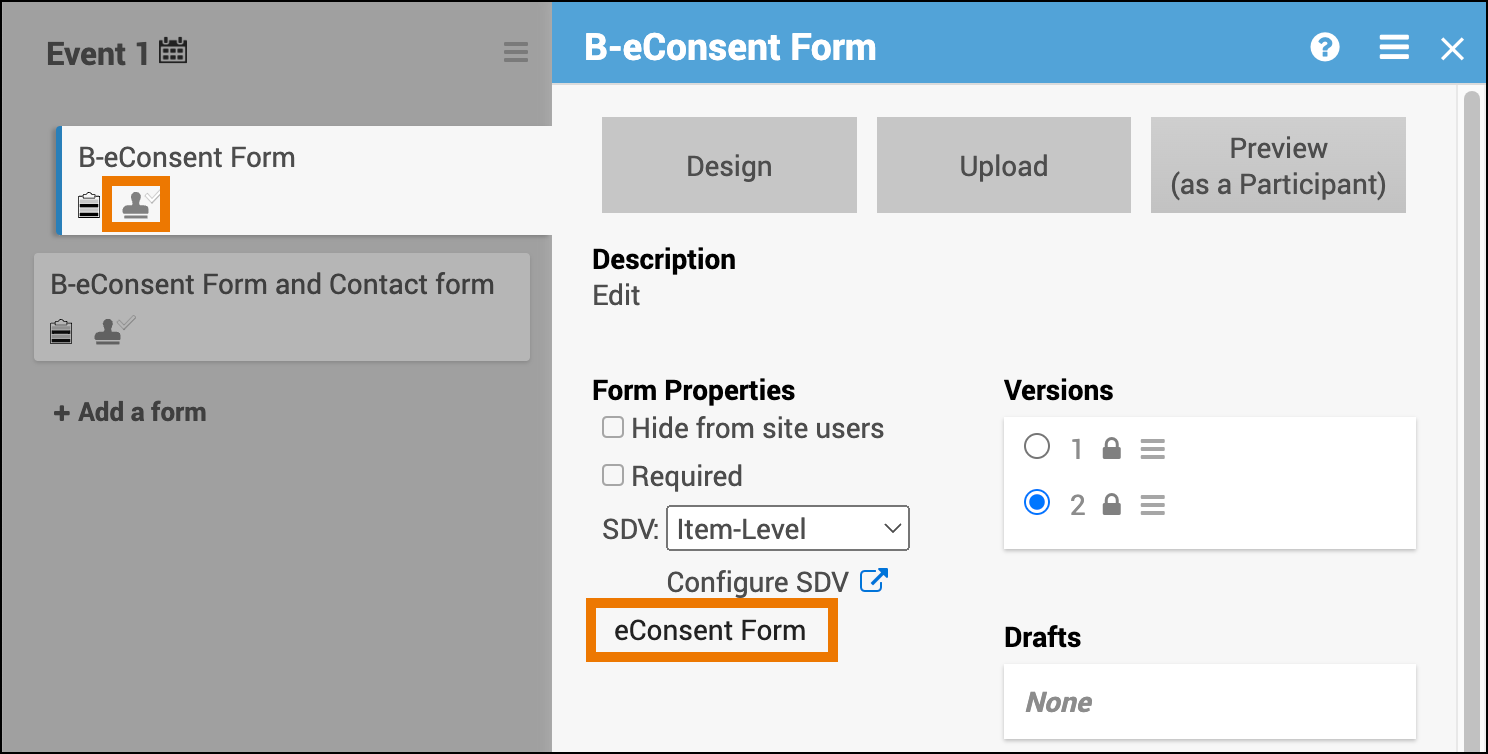
Once eConsent has been activated, Data Managers can designate Forms within non-repeating Visit Events as eConsent Forms using the Form Template. eConsent Forms are not available for use in Common Events or repeating Visit events.
Configure an eConsent Form:
- Using the latest Form Template, configure the form to have a multi-select “signature” checkbox item type with only one response with “1” as the response name:
- Survey Tab
- “select_multiple EC” in the type column
- “signature” in the bind::oc:external column
- The item cannot have a value in the bind::oc:itemgroup column
- Any item name and label can be used
- No values can be entered for required or readonly
- Choices Tab
- Use the list_name that matches the name defined in the Survey Tab’s type column (“EC” in the example above)
- Only one choice can be added for the item because we only want one checkbox to appear.
- The choice name must be “1” (without the quotation marks)
- Anything can be entered for the choice label; this is what will appear next to the checkbox as the prompt for the participant to click and sign.
- Survey Tab
- Make additional changes to the form definition as necessary, then upload the file to a Non-repeating Visit event. (Other event types do not support eConsent forms).
- After the form is successfully uploaded, the system will automatically update the Form Card to show that the form is an eConsent form.
- This cannot be edited within the Form Card or Study Designer, but would need to be changed by editing the form definition and re-uploading the form version
Note: Contact Data items and permission tags will work as normal, with the only exception being that all users will be able to see the eConsent status on the Participant Matrix. Queries cannot be added to signature checkbox items.
