After the eConsent module has been activated for your study and at least one eConsent form has been configured for it, Study Runner can be used to monitor participant consent statuses and schedule eConsent events to make the consent forms available to participants.
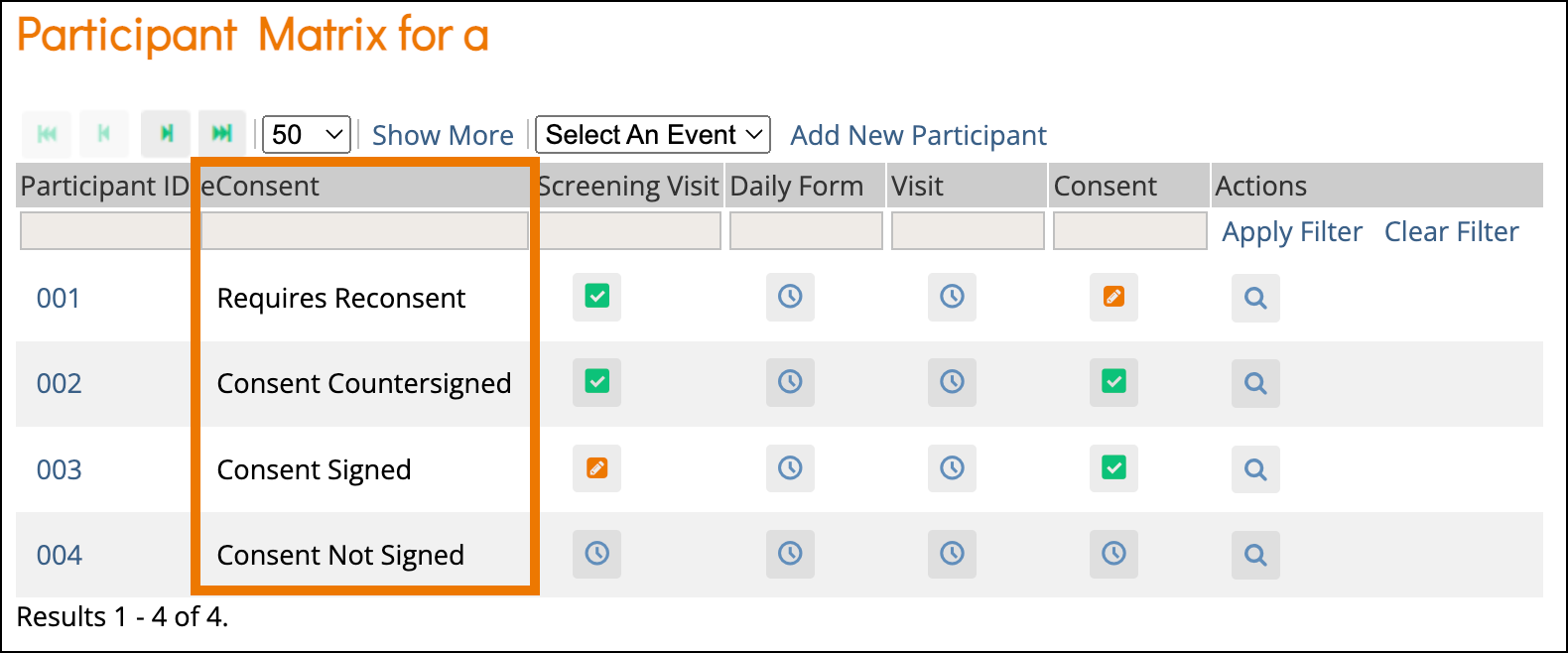
The eConsent status for all participants can be viewed on the Participant Matrix. A separate column will be displayed for each eConsent form in the study.
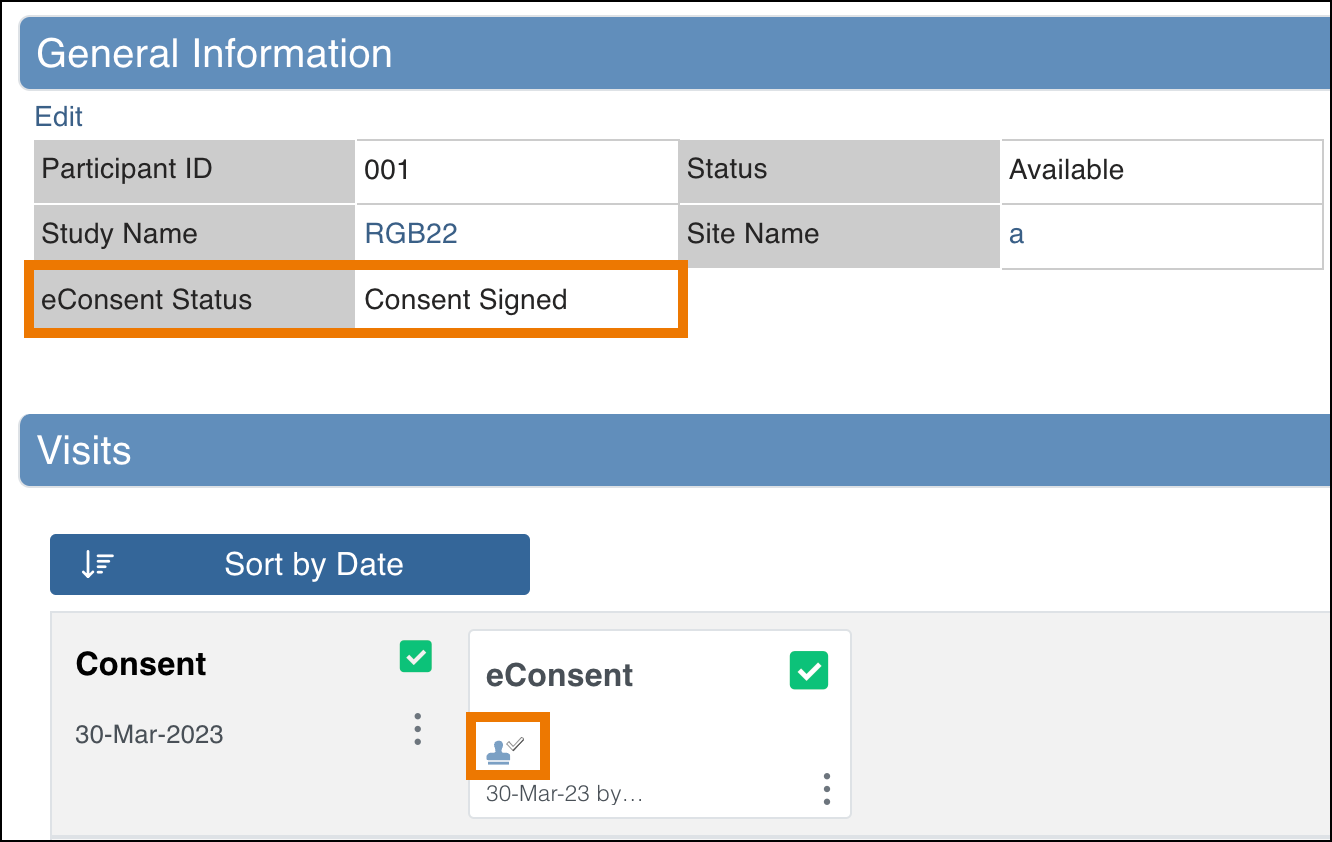
The eConsent status for individual participants’ eConsent forms can be viewed on the Participant Details Page both in the General Information section as well as on the form cards in the Visits section.
The eConsent statuses include the following:
| Icon | Status | Description |
 | Consent Not Signed | The participant has not yet signed this eConsent form. |
 | Consent Signed | The participant has signed this eConsent form, but it has not yet been countersigned by a site user (CRC or Investigator). |
 | Consent Countersigned | The participant has signed this eConsent form and it has been countersigned by a site user (CRC or Investigator). |
 | Requires Reconsent | This eConsent form was signed by the participant (and possibly also countersigned), but a site user (CRC or Investigator) removed the consent. This might be done if a newer version of the eConsent form is being used. The participant will need to sign for consent again. |
Schedule an eConsent Form:
For a participant to see the eConsent form on their dashboard, the event with the eConsent form will need to be scheduled first. This event is scheduled in the same way as any other Visit event is scheduled and can be done from the Participant Matrix or by using the Add New button on the Participant Details Page.
Invite the Participant:
The participant will need to be invited to the study to have access to the eConsent form.
Once the Visit with the eConsent form is scheduled and the participant has been invited to the study, the participant can sign the eConsent form using their dashboard. After the participant signs the eConsent form, the form will be marked as Completed and its eConsent status will be updated in Study Runner.
View an eConsent Form:
After a Visit event with an eConsent form has been scheduled for the participant, the form can be opened by any user with permission to view it, but only Participant users will be able to click the checkbox to mark the eConsent form as Signed. Open the form by clicking on the Visit form card or use its actions menu.
When viewing the eConsent form, the top of the page will display the information about when it was signed with the name of the participant (name included only when viewing as a CRC or an Investigator). The form will also display the information on who countersigned and when that signature was added.
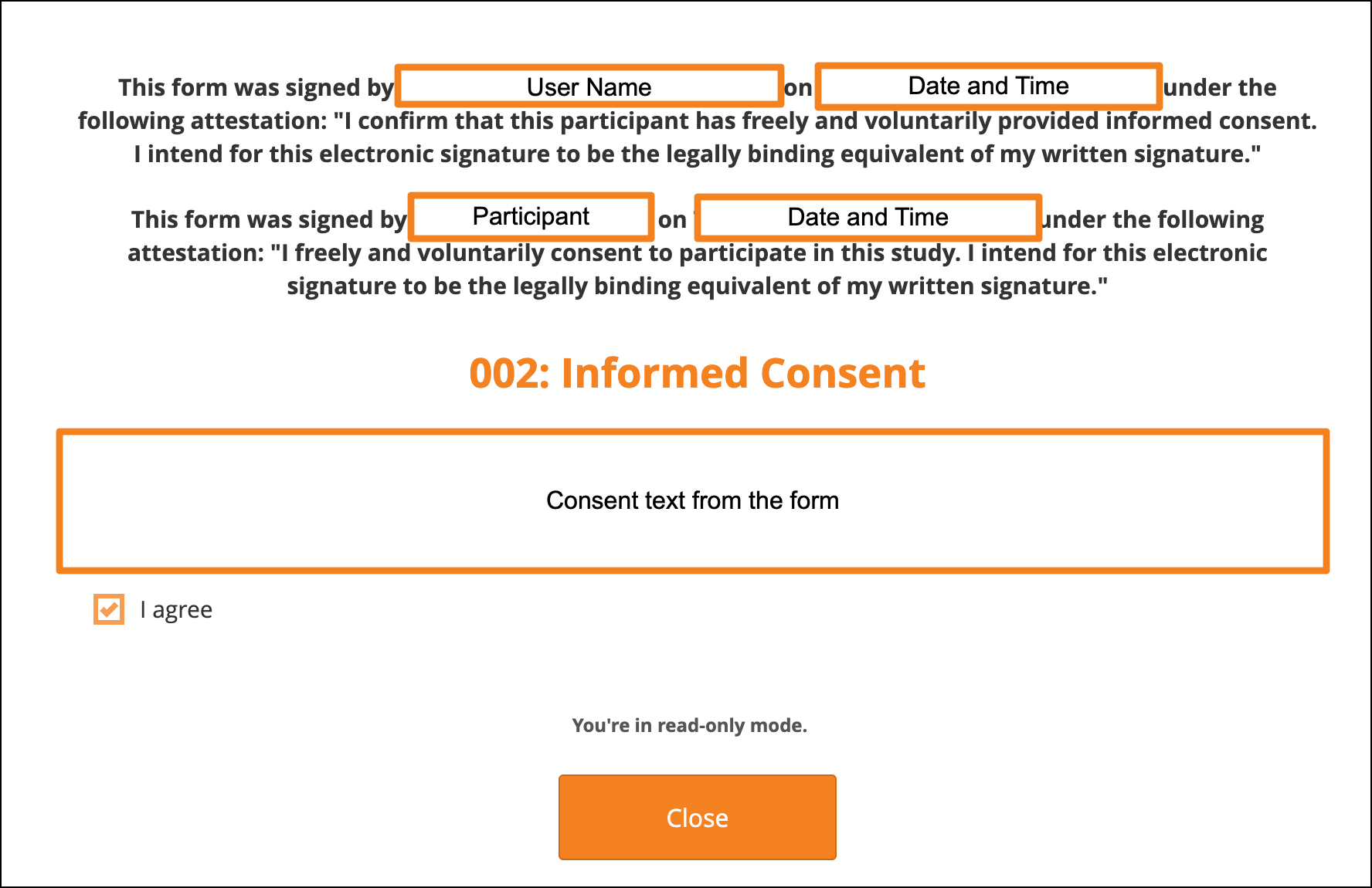
Note: The consent checkbox will be read-only. Only Participant users can click the checkbox.
Countersign a Signed Consent:
After the participant has signed their consent, the form’s eConsent Status will be updated accordingly on the Participant Matrix and on the Participant Details Page. Clinical Research Coordinators and Investigators can then countersign the form.
- Click the Actions menu on the signed eConsent form card and select Countersign.
- Enter your Username and Password to confirm your countersignature and click Submit.
- The eConsent status on the form card will be updated to Consent Countersigned.

Unconsent a Signed Consent:
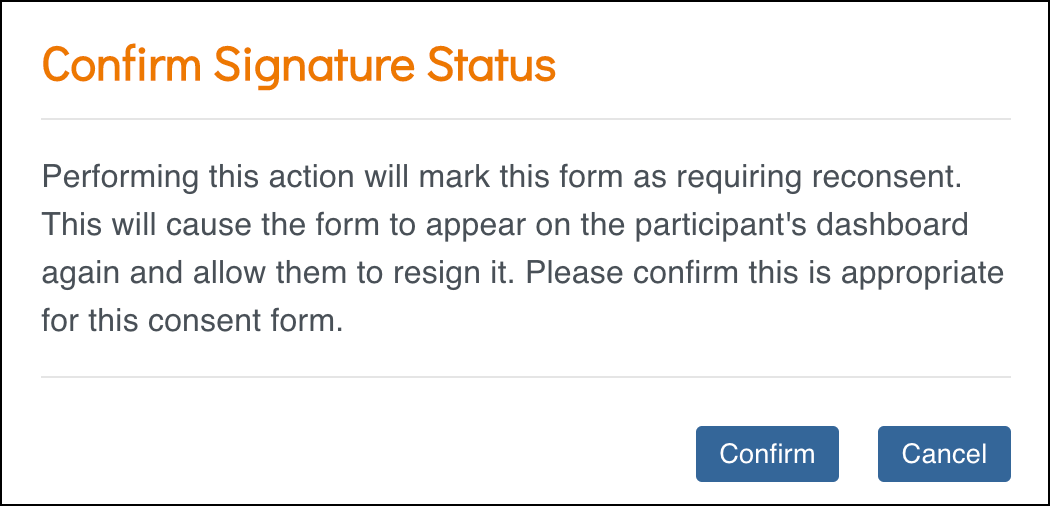
- Click the Actions menu on the signed eConsent form card and select Unconsent.
- The Confirm Signature Status window will open and explain:
- Performing this action will mark this form as requiring reconsent. This will cause the form to appear on the participant’s dashboard again and allow them to resign it. Please confirm this is appropriate for this consent form.
- Click Confirm to Unconsent the Signed Consent.
- The eConsent status on the form card will update to Requires Reconsent and the form status will be changed from Completed to Data Entry Started.
Note: The eConsent status in the General Information section of the Participant Details Page will update when the page is refreshed.
