Data Managers can activate Participate. Participate allows participants to enter data into online Forms at scheduled times.
To Activate Participate:
- On the My Studies screen, in Study Designer, or the on the Share screen, click the Settings (Gear) button, and select Modules.
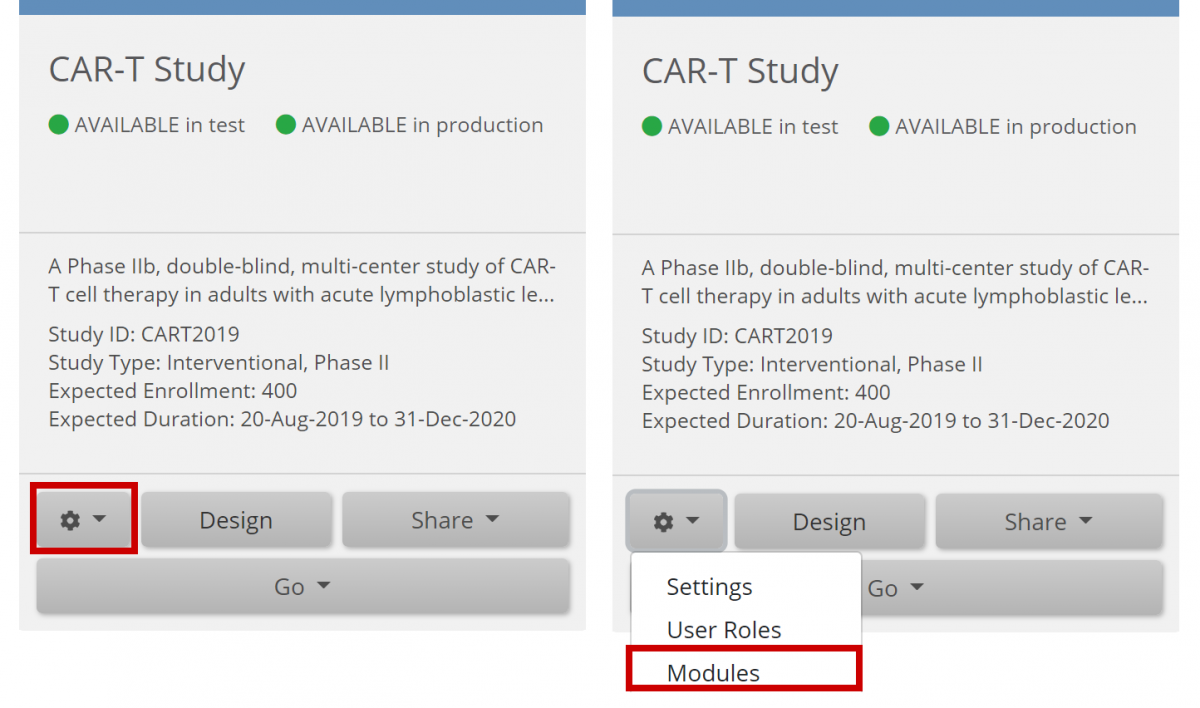
- Click the Request Access button on the Participate Module card.
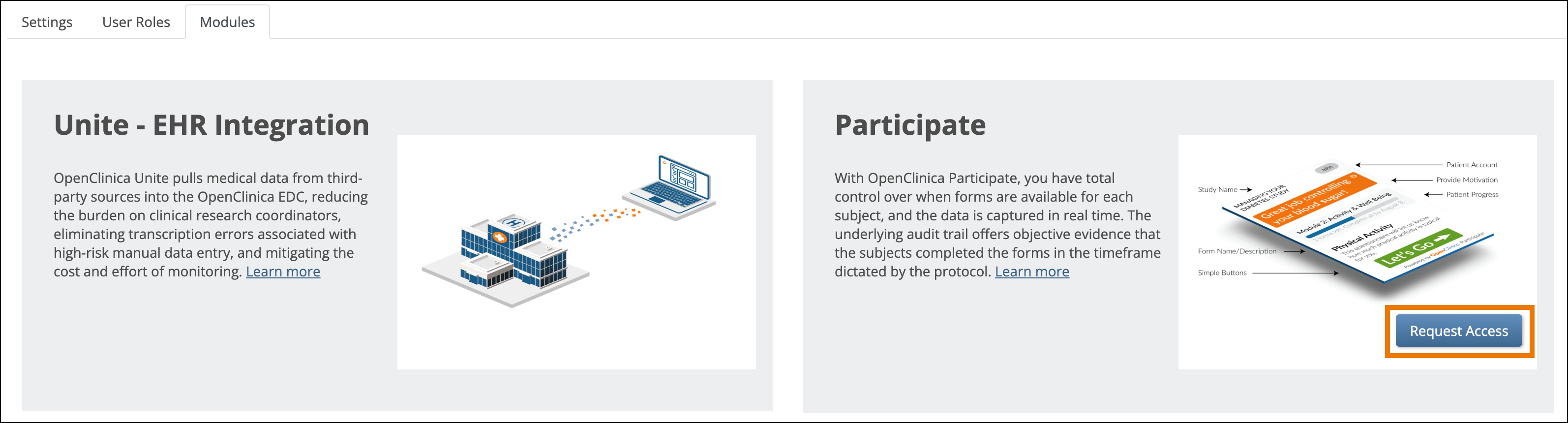
- Read the instructions in the popup, and provide a subdomain name for your study. The subdomain name can contain letters, numbers, and hyphens (-), but cannot begin or end with a hyphen.
The system checks to ensure that the subdomain is valid and is not already in use (subdomain names are case insensitive, so a subdomain of Juno is equal to a subdomain name of juno and would therefore be considered already in use).
If you do not receive a message indicating that your request was successfully submitted, check the subdomain name you provided and try again.
Note: Once you have requested access, the status of the Participate module is set to Pending. While it is in this status, you can start designing Participate Forms, but they are not activated for Participant use until the request is approved and the status is set to Active. Requests are approved by OpenClinica Customer Support based on the current Participate contract with your organization.
Once your Participate request is approved, the status is set to Active, and Participate is fully available for use in the Test and Production environments for that study.
To deactivate Participate at any time, return to the Module Management screen, and click the Deactivate button under the Participate module card. If you confirm the deactivation, this removes all Participant access to Forms and reverts all Participate Form settings, but all Participate forms remain accessible to OpenClinica users.
