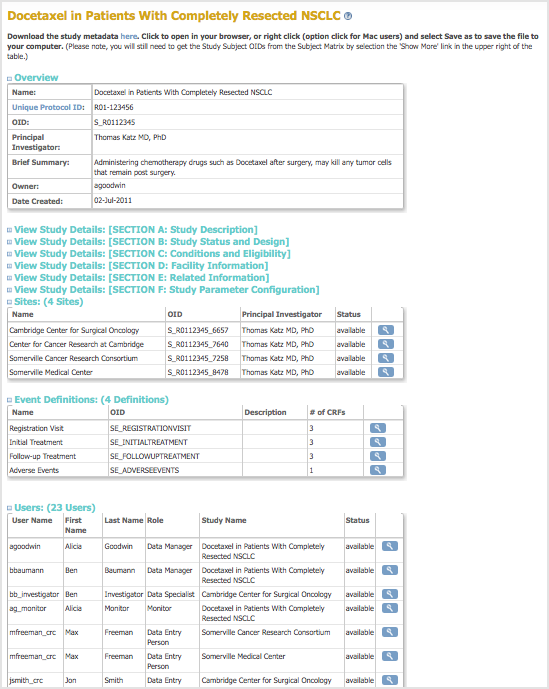
To see the configuration for the current Study or Site, and the users assigned to it, select Tasks > View Study. The page displays the configuration details for the Study or Site. Following are the tasks you can perform from the page, if your user Role has appropriate permission:
- Download Study Metadata: At the top of the page is a link to the Study metadata. Click the link to download the Object Identifiers (OIDs) for the Study. The OIDs are required for Data Import and for creating Rules in an XML-formatted file, and can be useful when extracting data to files that use certain formats.
- View Definitions: When viewing the tables on this page, click any of the links in the Name column to view the definition at the clinicaltrials.gov website.
- Overview: The Overview section presents information about the overall Study or Site.
- View Study Details (Sections A to F): Click the link for any section to view details for it.
- Sites: When the current Study or Site is set to the Study level, the Sites section shows all Sites in the Study. For details about a Site, click the View icon.
- Event Definitions: Lists the Event Definitions. Click the View icon for an Event to see all CRFs for it, and to access CRF details.
- Users: Lists the users assigned to the Study or Site. Click the View icon for a user to access more information.
For more information any of the items on the View Study page or how to change them, see Build Study.
View Study Page. The Unique Protocol ID item is a link that displays a definition when you click it: