Study-level Data Managers and Administrators can request the eConsent module for activation. eConsent allows users to get signatures from participants and countersignatures from Investigators and CRCs through electronic means.
Request eConsent
- On the My Studies screen, in Study Designer, or the on the Share screen, click the Settings (Gear) button, and select Modules.
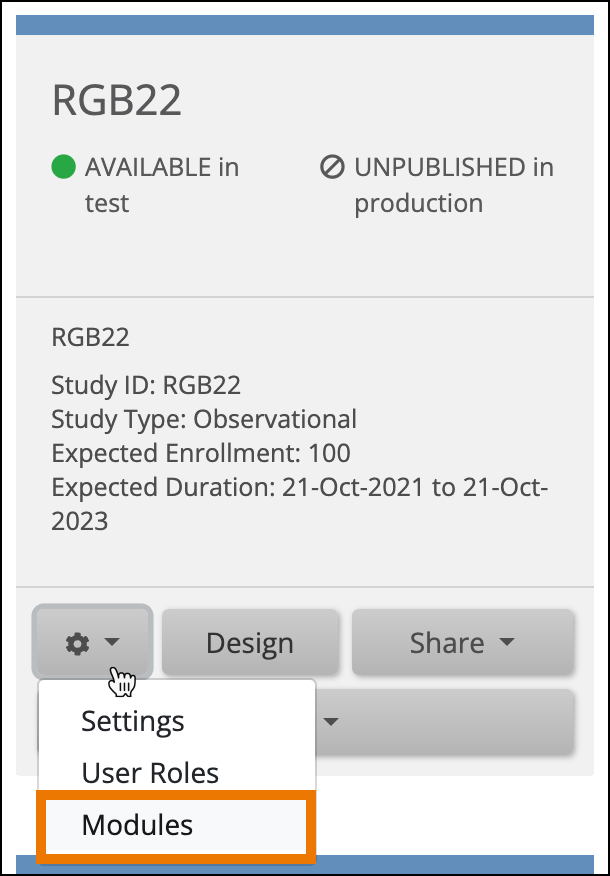
- Click the Request Access button on the eConsent Module card.

Note: Once you have requested access, the status of the eConsent module is set to Pending. While it is in this status, you can start designing eConsent Forms, but they are not activated for use until the request is approved and the status is set to Active. Requests are approved by OpenClinica Customer Support based on the current eConsent contract with your organization.
Once your eConsent request is approved, the status is set to Active, and eConsent is fully available for use in the Test and Production environments for that study.

