5.3.1 About Study Details
The following information can help you complete or edit values in the Update Study Details page.
All Sections
- When a field name is a link (blue text), you can click it to see the definition at the clinicaltrials.gov website.
- Information you provide in all sections except Study Parameter Configuration is for reporting and does not affect data collection. For example, if you specify a minimum age, OpenClinica does not use that information to verify the age of a Subject you add to the Study. The information you provide in Study Parameter Configuration does impact data collected in OpenClinica for the Study. For example, if you specify Yes for Collect Subject Date of Birth, then the full date of birth must be entered when adding a Subject to the Study.
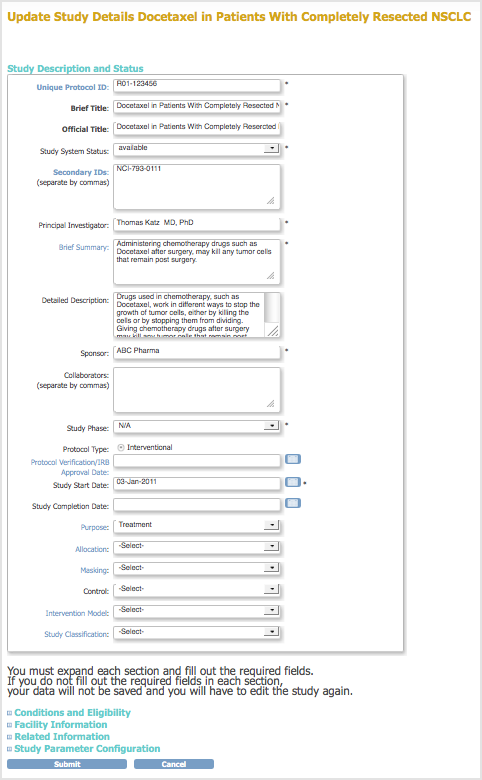
Study Description and Status Section
- Unique Protocol ID is the user-defined number assigned to the Study when it was first created in OpenClinica.
- You cannot modify the Study System Status on the Update Study Details page. For more information, see Status of a Study.
- Principal Investigator is the person running the Study, and is responsible for the Study being completed and performed correctly.
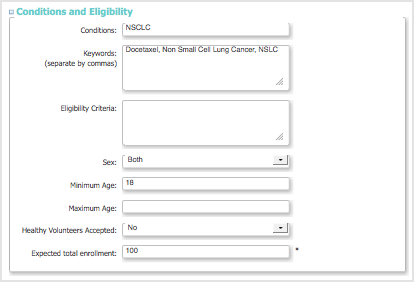
Conditions and Eligibility Section
- This section can be used as a guideline for conditions and eligibility for the Study.
- Expected total enrollment is used for reporting statistics. It does not limit how many Subjects can be added to the Study, nor is it treated as a required minimum.
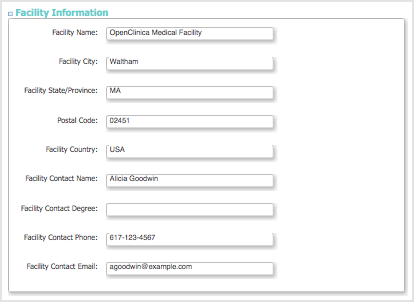
Facility Information Section
- Facility information can be for the sponsor, or a main location for the Study. There can be multiple facilities within a Study, where each is defined as a Site after the Study has been created.
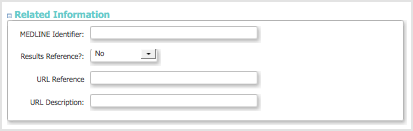
Related Information Section
- The URL Reference and URL Description can be used to indicate where you publish your Study results.
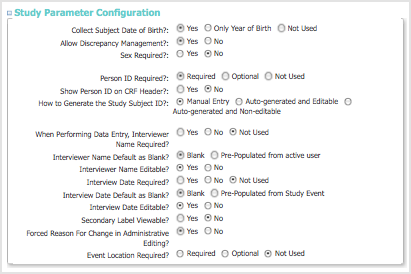
Study Parameter Configuration Section
- If ‘Collect Subject Date of Birth’ parameter is set to ‘Only Year of Birth’, then the date of birth will be stored internally as January first of the year.
- If you specify No for Allow Discrepancy Management, you cannot create Notes and Discrepancies for CRF Data for the Study.
- If you edit the Sex Required field to change it from Yes to No, the item still appears in the CRF header but completing the field is not required.
- You can specify whether or not the Person ID is required in OpenClinica release 3.1 and higher. In releases prior to 3.1, you specify the parameter using show_unique_id for global ID in the datainfo.properties configuration file.
- When you specify Interviewer Name Required, and Interview Name Pre-Populated from active user, the active user is the user who scheduled the Event.
- When you specify Yes for Forced Reason For Change in Administrative Editing, a Discrepancy Note will be required to modify any Item in a CRF after that CRF has been marked complete.
- The ‘Location’ field can be used when scheduling or editing a study event. If set to ‘Not Used’ it will not appear in the user interface for the study.