When you sign an Event, you provide your approval of all CRF data for the Event for the Subject. To sign an Event:
- Follow the instructions in Manually Change Event Status, and set the status to “signed.”
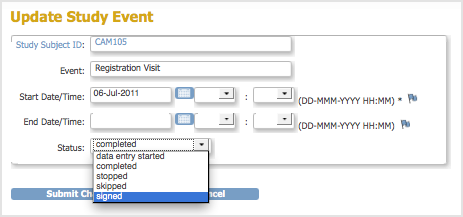
- Click Submit Changes.
The Update Study Event page displays links to all information for the Subject for the Study; click a link to review the information. It also presents a statement about what signing the Subject record signifies.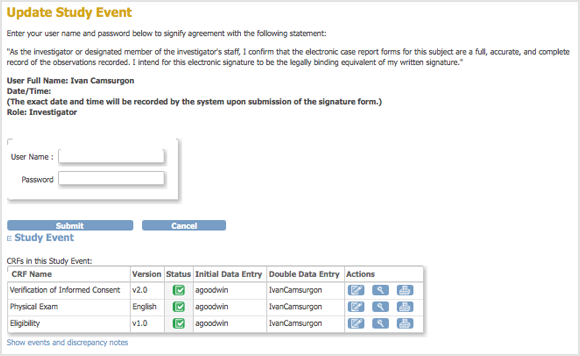
- Enter your user name and password in the appropriate fields.
- Click Submit.
The View Subject page opens, showing the status for the Event as “signed.”
