This article describes a method for downloading annotated CRFs (aCRFs) which are automatically available for Data Manager roles within Study Runner. Annotated CRFs can be downloaded individually or an Annotated Casebook can be downloaded containing all CRFs for the entire study.
Download Individual Annotated CRFs
- From the Tasks menu in Study Runner, select CRFs.
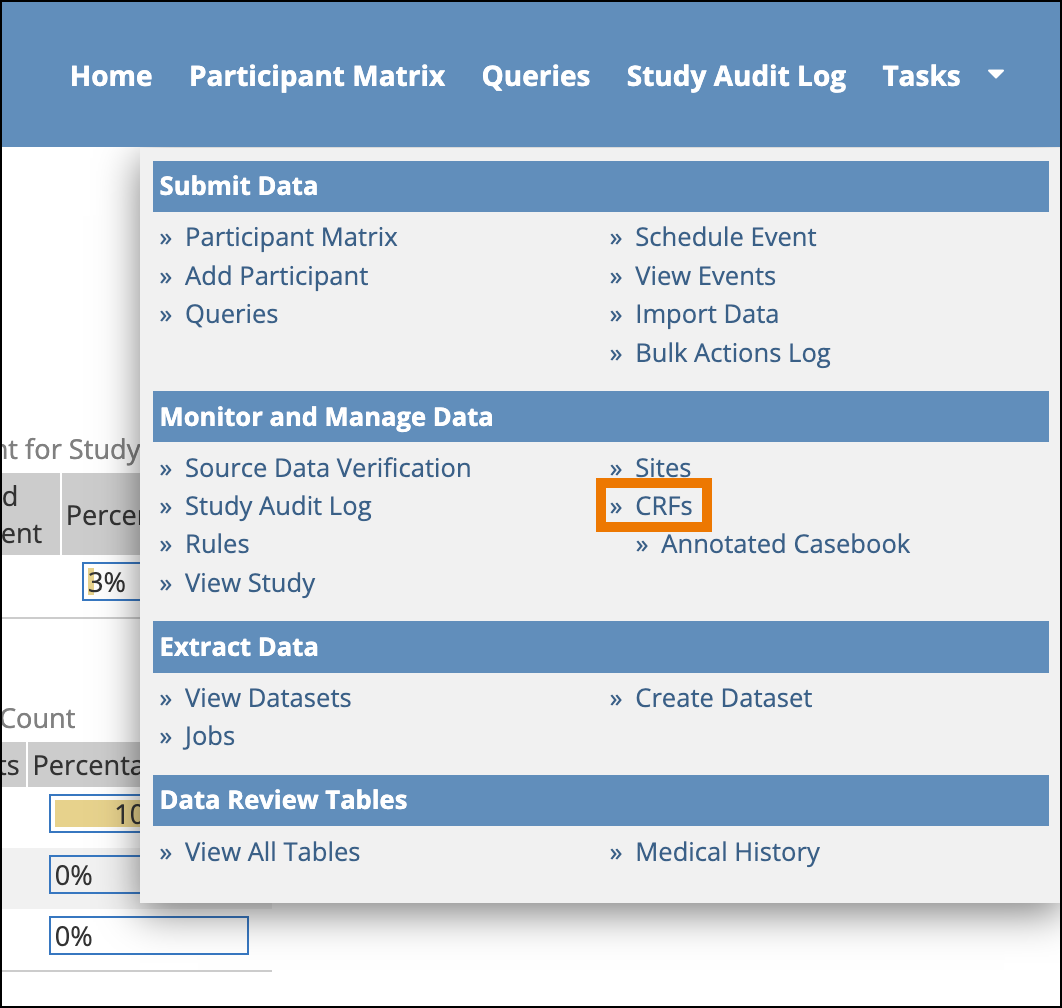
- The Manage Case Report Forms (CRFs) page opens. Click the Download icon to download an individual Annotated CRF for the form version on that row.
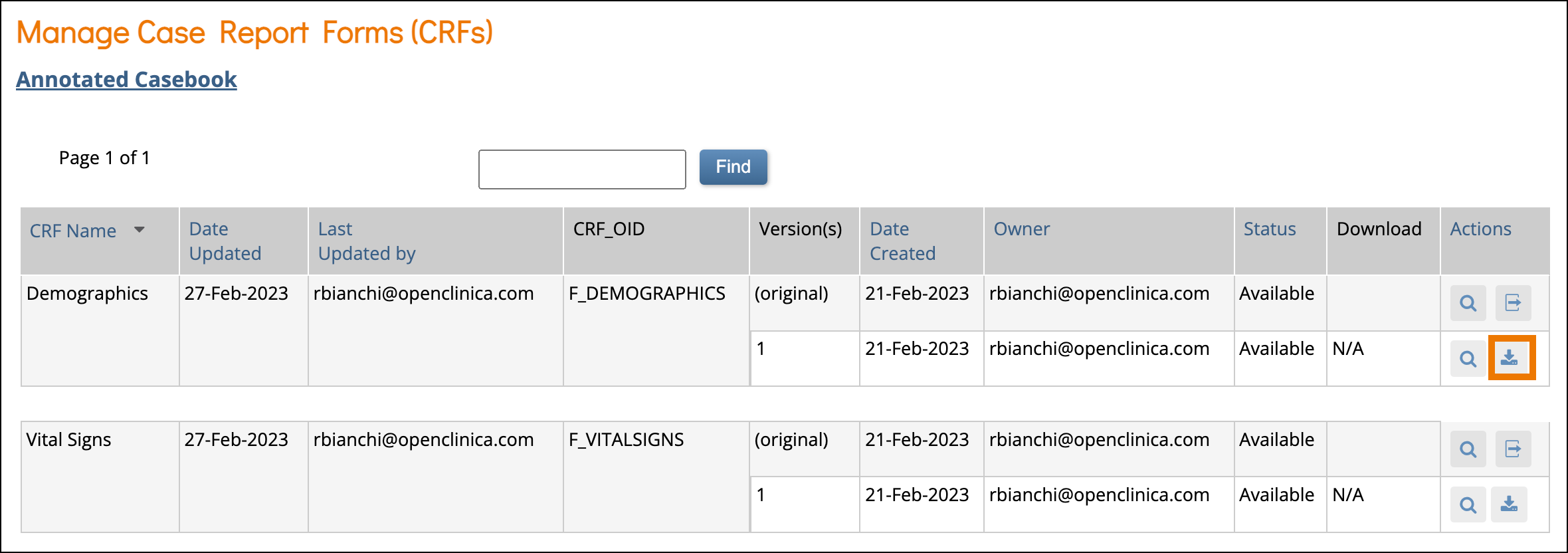
The downloaded PDF document will include a cover page with Study Name, Study OID, Form Name, Form OID, Form Version, and Form Version Initial Publish. It also includes the generated date and time in the footer.
While there are labels for the Event Name/OID and SDV Configuration for individual CRFs, the values are not displayed because the form could be in more than one event. Download the Annotated Casebook for the study to view these values. From the Manage Case Report Forms (CRFs) page, you can use the Annotated Casebook link to get all Annotated CRFs for the study, or follow the steps in the next section.
Download the Annotated Casebook for the Study
The Annotated Casebook compiles the default versions of active forms in the study, collates them by event, and creates a PDF you can download. Each item includes annotations that describe the form’s configuration. This option is only available to Study Data Manager roles and is only available when accessing the study at the Study level (the option is not available when logged in at the Site level).
- From the Tasks menu in Study Runner, select Annotated Casebook.
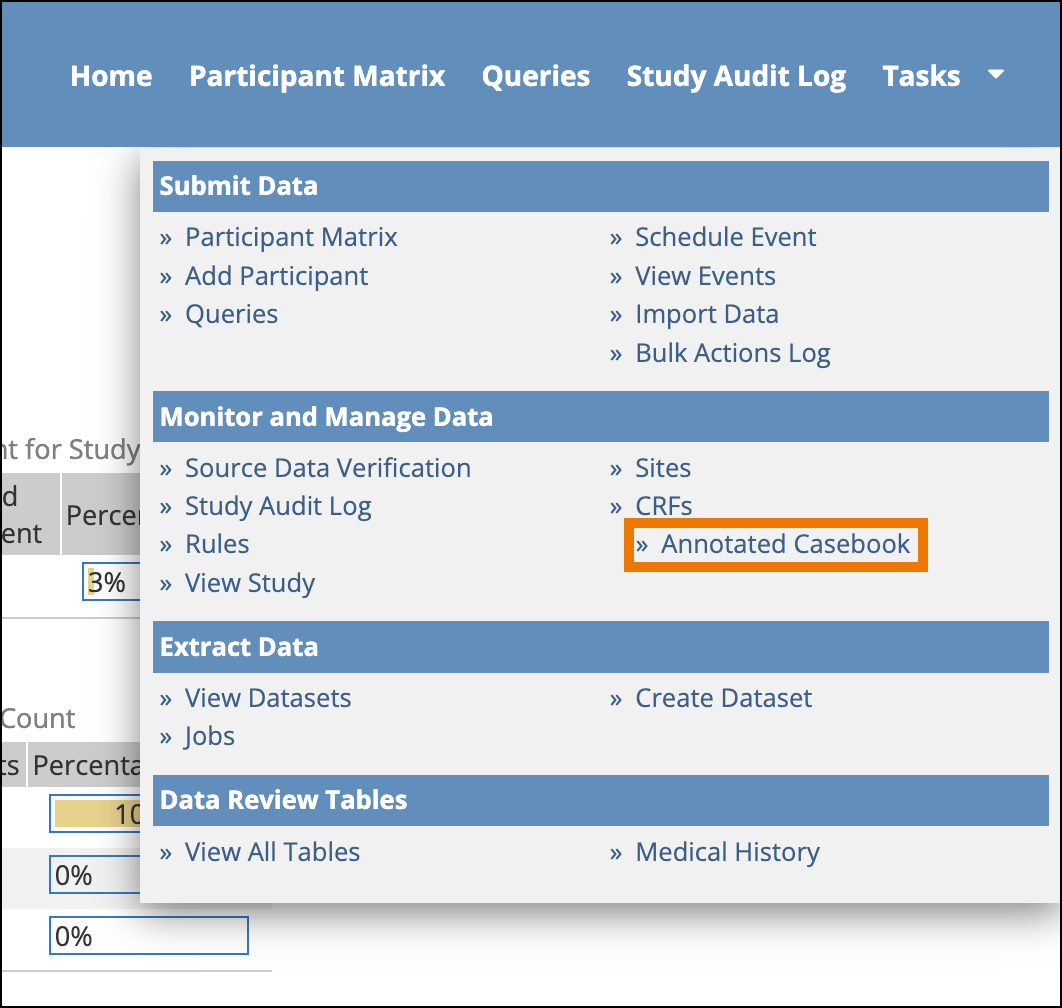
Note: The Annotated Casebook page can also be accessed by clicking the Annotated Casebook link on the Manage Case Report Forms (CRFs) page.
- Select the Include archived events and forms checkbox if you want to include these in the PDF, otherwise only Active forms will be included by default. Click Get PDF.

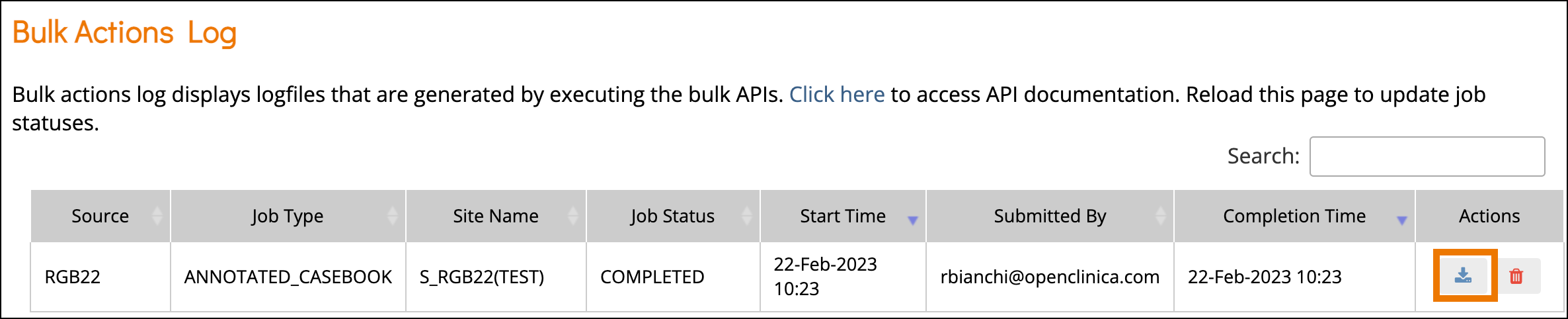
- A green message bar will appear letting you know that the PDF is being generated. This may take some time if it is a large study. Click the Bulk Actions Log link to navigate to the Bulk Actions Log and view the status of the job.
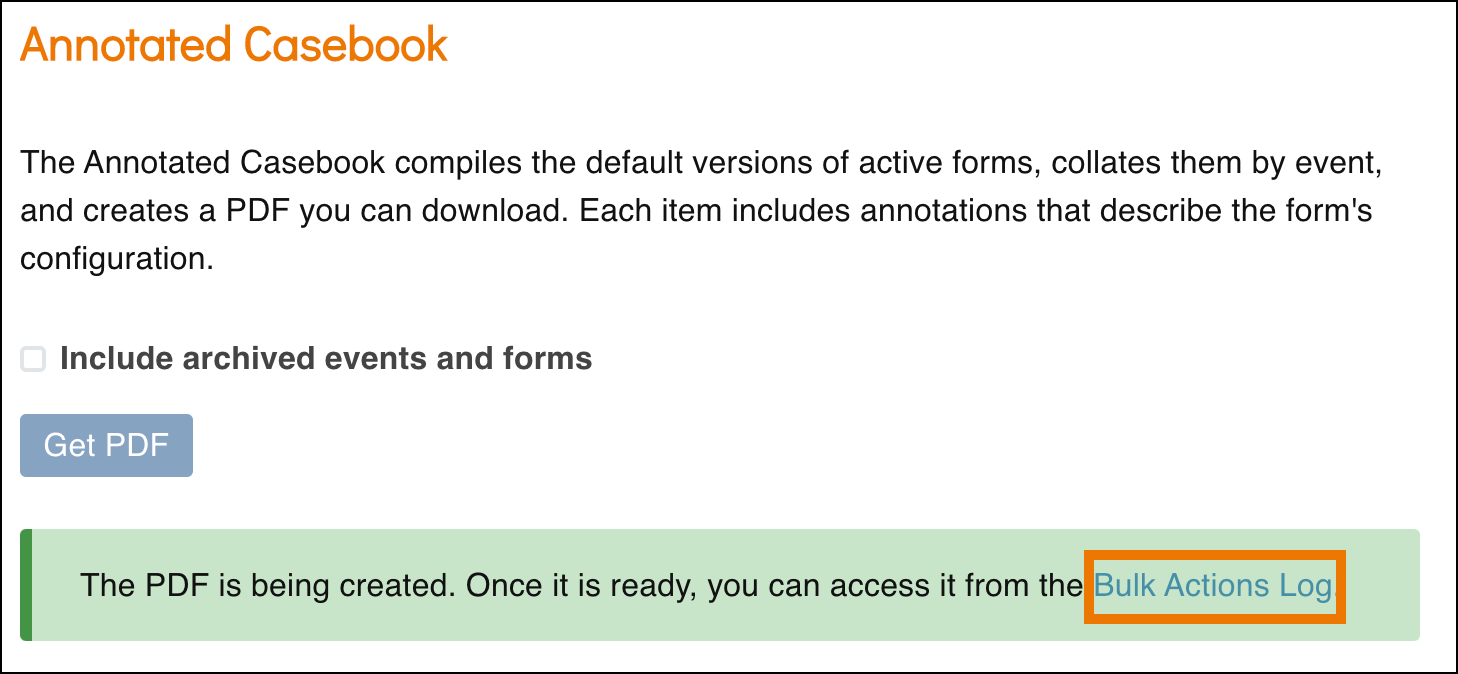
- Click the Download icon to download the Annotated Casebook PDF that was generated.
The Annotated Casebook includes the date and time it was generated and the page number on each page. It also includes:
- A cover page with the name of the study, the date and time it was generated, and the page numbers.
- A Table of Contents that includes the name of the study and the study OID, a heading for each event and a page number for each CRF below the associated event.
- A title sheet for each aCRF which includes Study Name, Study OID, Event Name, Event OID, Form Name, Form OID, Form Version, Form Version Initial Publish and SDV Configuration.
- An annotated CRF for each CRF included in the download.
Reviewing Annotated CRFs
When reviewing Annotated CRFs, text will appear in different colors to help differentiate the annotations. Each annotation is based off the form definition with similar names. Below is a list of the Annotation Labels, their associated names on the Form Definition, the color and the order which they will appear.
| Order in aCRF | Annotation Label | Form Definition Name | Color |
|---|---|---|---|
| 1 | Label | label | black |
| 2 | Name | name | orangered |
| 3 | Type | type | black |
| 4 | Item Group | bind::oc:itemgroup | blue |
| 5 | Show When | relevant | green |
| 6 | Required | required | red |
| 7 | Required Type | bind::oc:required-type | cornflowerblue |
| 8 | Constraint | constraint | magenta |
| 9 | Default | default | deepskyblue |
| 10 | Calculation | calculation | maroon |
| 11 | Trigger | trigger | darkgreen |
| 12 | Read-Only | readonly | chocolate |
| 13 | Image | image | darkviolet |
| 14 | Repeat Count | repeat_count | lime |
| 15 | External | bind::oc:external | indigo |
The Annotated values occasionally need to have some symbols changed from their value on the Form Definition to their value on the Annotated CRF. Below is a list of the original values from the Form Definition to the replacement value that will appear on the Annotated CRF along with each Annotated Label which may have that symbol change.
| Original Symbol | Annotation Replacement Symbol | Annotation Label |
|---|---|---|
{ | [ | Default, Calculation, Label, Required, Constraint, Trigger, Item Group, Choice Name, Choice Label, Show When |
} | ] | Default, Calculation, Label, Required, Constraint, Trigger, Item Group, Choice Name, Choice Label, Show When |
> | gt | Default, Calculation, Required, Constraint, Trigger, Item Group, Show When |
< | lt | Default, Calculation, Required, Constraint, Trigger, Item Group, Show When |
Note: Any label formatting done in the form definition will carry over to the annotated CRF. For example, if the label includes *label* then the label will be bolded.
Languages
Annotated CRFs and Casebooks can be displayed in different languages (only for studies configured to support this). When a form has multiple languages configured, the annotated CRF or Casebook will display in the same language that would appear for the user when loading opening a form to edit on screen.
This only applies to the labels and hints defined for the form. Annotations are always in English as described above.
Note: OpenClinica does not yet have every language configured and tested. New languages are added to the system by request. If your preferred language is not currently set up in OpenClinica, please reach out to our Support team to discuss getting it added.
