3.2.1 Audit Logs Page for a Subject
To view the Audit Logs of Events for a Subject, click the View icon in the Actions column of the View Study Log page. The Audit Logs page opens for the Subject, providing a detailed history for all Events for the Subject.
You can also access the Audit Logs for a Subject from the Subject Matrix. Click the View icon in the Actions column of the Subject Matrix, and then on the View Subject page, expand the Study Subject Record section and select the Audit Logs link (located at the top of the section). The Subject’s Audit Logs page opens.
Excerpt from Audit Logs Page for a Subject (CAM103):
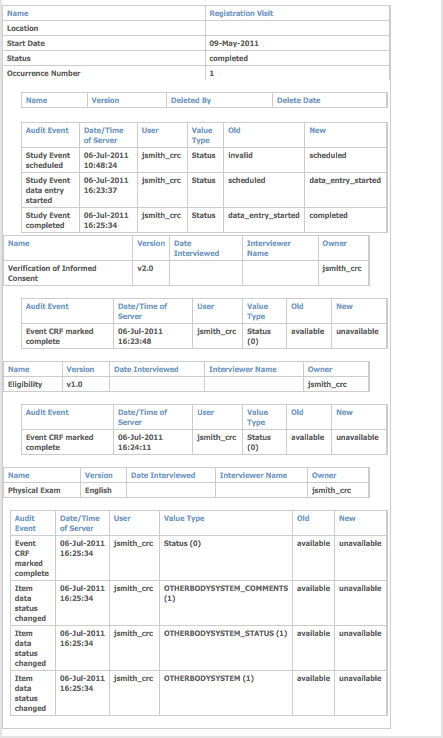
Download the Audit Log to Excel
To download the Audit Log to Excel, click the blue download button to the right of the Audit Log header.
Then, to open the Audit Log in Excel, click OK.
The Subject information is displayed on the first worksheet, and the audit information for each Event is displayed on a separate worksheet.
Note: Though the Audit Log may be easier to read in Excel, this cannot be considered a compliant Audit Log since it can be edited. This functionality is solely provided for ease of viewing the audit information. The Audit Log that is viewed in OpenClinica, however, is fully compliant and cannot be edited.
3.2.2 What the Study Audit Log Tracks
The Audit Log tracks the following for Events:
- Date and time
- Location
- Status
- Date started
This table lists the actions the Audit Log tracks:
| What | Actions |
| CRF | Initial data entry complete |
| CRF | Initial data entry completed with password |
| CRF | Double data entry complete |
| CRF | Double data entry completed with password |
| CRF | Marked complete |
| CRF | Completed with password |
| CRF | Properties changed |
| CRF | Source data verification status changed |
| Item on CRF | Data value updated (see Note, below the table) |
| Item on CRF | Data status changed |
| Item on CRF | Data deleted |
| Item on CRF | Data inserted for repeating row |
| Event | Scheduled |
| Event | Data entry started |
| Event | Completed |
| Event | Stopped |
| Event | Skipped |
| Event | Locked |
| Event | Removed |
| Event | Start date changed |
| Event | End date changed |
| Event | Location changed |
| Event | Signed |
| Study Subject | Created |
| Study Subject | Status changed |
| Study Subject | Value changed |
| Subject | Created |
| Subject | Status changed |
| Subject | Value changed |
| Subject | Global value changed |
| Subject | Site assignment made |
| Subject | Group assignment made |
| Subject | Group changed |
Note: Starting with the OpenClinica 3.1.2 release, initial values for CRF Items provided during data entry are tracked in the Audit Logs, and reported as “Item data value updated.” This is only tracked when a value is provided; empty or non-existent values are not tracked. Prior to the OpenClinica 3.1.2 release, initial values for CRF items were not tracked.
The Audit Log does not track Discrepancy Notes, but you can view or download the full thread for any Discrepancy Notes you want to see. Discrepancy Notes cannot be removed, so they are all always available.

