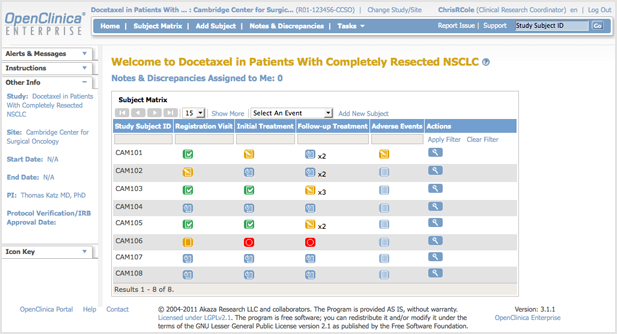
1.2.1 Top of Page
The top of the page is the area above the navigation bar. It contains:
- Name of Current Study: The name of the current Study, and the current Site if one is selected. To view more information about the Study or Site, click the name. For more information, see About Study Details.
- Change Study/Site: Allows you to change the current Study or Site. See more details about changing the Study or Site.
- User Name and Role: Name of the user who is currently logged in to OpenClinica and the user Role they are assigned to in OpenClinica, such as Data Manger.
- Log Out: Ends your OpenClinica session. See more about logging out.
1.2.2 Navigation Bar
The navigation bar, which is near the top of the page, provides access to the main features in OpenClinica. The features available depend on your user role.
- Home: Wherever you are in OpenClinica, click Home to go to the home page for the current Study or Site.
- Subject Matrix: The Subject Matrix is a table with Event information for all Subjects in the current Study or Site. You can view, enter, and change information for Subjects and their Events in the Study. There is one Subject per row, with the Study Subject ID in the first column. The other columns are for each Event Definition in the Study. Each cell contains a colored icon that identifies the status of Event(s) for the Study Subject. Move the cursor over an icon in the matrix to view and access a Subject’s data and actions for that Event. Refer to the Icon Key in the sidebar for icon descriptions. See more details about the Subject Matrix.
- Add Subject: Allows you to add a new Subject to the Current Study or Site.
- Notes & Discrepancies: Notes and Discrepancies provide query and discrepancy management capabilities to help you verify the accuracy and completeness of data in a clinical trial. Use this set of tools to flag and track issues that arise when capturing, monitoring, and managing data in a Study (such as incomplete or inaccurate data), and to facilitate communication between users (such as Clinical Research Coordinators and Monitors) to resolve these issues. See more details about Notes and Discrepancies.
- Study Audit Log: The Study Audit Log provides a history of actions for subjects in the Study. See more details about the Study Audit Log.
- Tasks: Click Tasks for a menu of actions you can perform in OpenClinica. The actions are grouped into modules in the Tasks menu. Modules and items included in the Tasks menu depend on permissions for your user Role.
- Report Issue: Click the Report Issue link when you experience what you believe is a problem with OpenClinica. See more details about reporting issues.
- Support: Click Support to access support information. See more details about getting support.
- Search Box and Go Button: To view a history of Events for a Subject and access other information about the Subject, enter the Study Subject ID in the search box; then click Go or press Enter.
1.2.3 Sidebar
The sidebar is a set of panels along the left edge of the page. To expand a panel (show information in it), click the down arrow in the panel. To collapse a panel (hide information in it), click the minus sign.
- Alerts and Messages: These alerts and messages display when you perform actions in OpenClinica. For example, after you log in, a message reports the date you last logged in. Look here for confirmation that an action you performed was successful, or to see if there were any errors.
- Instructions: The Instructions panel provides brief directions or a summary of what you can do at that point in OpenClinica. For more information, click the Help button (question mark) in the body of the page.
- Other Information: This shows the name of the current Study or Site, start and end date of the Study, the name of the Principal Investigator, and the Protocol Verification/IRB Approval Date, if it applies. To see details for the Study or Site, click the name. For more information, see About Study Details.
- Icon Key: The panel is available when icons are used in the body of the page, such as for the Subject Matrix. It shows each icon relevant to that page and describes what the icon means.
1.2.3.1 Status Icons
| Not started | |
| Scheduled | |
| Data entry started | |
| Stopped | |
| Skipped | |
| Completed | |
| Signed | |
| Locked | |
| Invalid |
1.2.3.2 Action Icons
The icons available and exactly what they do when you click them are based on your user Role.
| View: Click to see details for the object the icon is associated with | |
| Edit: Click to change details for the object. | |
| Set Role: Click to assign a Role for the user within the Study. | |
| Remove: Click to remove the object. | |
| Restore: Click the Restore icon to restore an object that was previously removed. (The icon appears only after object has been removed.) |
1.2.3.3 Complete Icon List
| Administrative editing | |
| Archive | |
| Calendar | |
| Completed | |
| Create new | |
| Delete | |
| Discrepancy Note: add | |
| Discrepancy Note: closed | |
| Discrepancy Note: new | |
| Discrepancy Note: not applicable | |
| Discrepancy Note: resolution proposed | |
| Discrepancy Note: updated | |
| Double data entry | |
| Download | |
| Edit | |
| Enter data | |
| Export | |
| Form contains changed/unsaved data | |
| Form has unchanged data | |
| Help | |
| Initial data entry completed | |
| Invalid | |
| Locked | |
| New version | |
| Not started | |
| Reassign | |
| Remove | |
| Remove role | |
| Restore | |
| Scheduled | |
| SDV complete | |
| Set role | |
| Sign | |
| Skipped | |
| Started | |
| Stopped | |
| Un-archive | |
| View |
1.2.4 Body of Home Page
What you see in the body of the home page is based on your user Role and the action you just performed. See more details about the body of the home page.
1.2.5 Bottom of Page
The bottom of the page contains:
- OpenClinica Portal: Click OpenClinica Portal to go to https://community.openclinica.com/. The portal provides valuable information and links to the developers workspace, getting started with OpenClinica, forums, news and upcoming events, a CRF library, and much more.
- Help: Click the Help link at the bottom of the page to open the OpenClinica User Documentation.
- Contact: If you want to send feedback or questions to the OpenClinica Business Administrator for your organization, click Contact, then complete and submit the form.
- Software License: OpenClinica is distributed under the GNU Lesser General Public License (GNU LGPL). The GNU Lesser General Public License is a free software license, and as such, grants to you the four following freedoms:
1.
The freedom to run the program for any purpose.
2. The freedom to study how the program works and adapt it to your needs.
3. The freedom to redistribute copies so you can help your neighbor.
4. The freedom to improve the program and release your improvements to the public, so that the whole community benefits.You may exercise the freedoms specified here provided that you comply with the express conditions of this license. The LGPL is intended for software libraries, rather than executable programs.
The principal conditions are:
You must conspicuously and appropriately publish on each copy distributed an appropriate copyright notice and disclaimer of warranty and keep intact all the notices that refer to this License and to the absence of any warranty; and give any other recipients of the Program a copy of the GNU Lesser General Public License along with the Program. Any translation of the GNU Lesser General Public License must be accompanied by the GNU Lesser General Public License.
If you modify your copy or copies of the library or any portion of it, you may distribute the resulting library provided you do so under the GNU Lesser General Public License. However, programs that link to the library may be licensed under terms of your choice, so long as the library itself can be changed. Any translation of the GNU Lesser General Public License must be accompanied by the GNU Lesser General Public License.
If you copy or distribute the library, you must accompany it with the complete corresponding machine-readable source code or with a written offer, valid for at least three years, to furnish the complete corresponding machine-readable source code. You need not provide source code to programs which link to the library.
Any of these conditions can be waived if you get permission from the copyright holder. Your fair use and other rights are in no way affected by the above.
For details see http://www.gnu.org/licenses/lgpl-2.1.html, or LICENSE.txt provided with the OpenClinica distribution.
- Version Number: The version number is for the OpenClinica software you are using.