Definition:
A Study uses Events and Forms to collect Participant data to determine the safety and effectiveness of a new treatment.
Example:
A Study could be a trial of a new medication intended to treat diabetes.
The Study Build System is where you design and set up your study. Runtime is where you collect and review data.
The process starts in the Study Build System and continues in Runtime.
Only users with a User Type of Administrator and a User Role of Data Manager can create a study. For information on User Types and User Roles, see User Access.
To create a Study:
1. With a User Type of Administrator and a User Role of Data Manager, log into the OpenClinica Study Manager.
2. To open the Add a new study window, scroll to the bottom of the screen, and click Add a new study.
3. Enter data in the fields that appear on the Add a new study window.
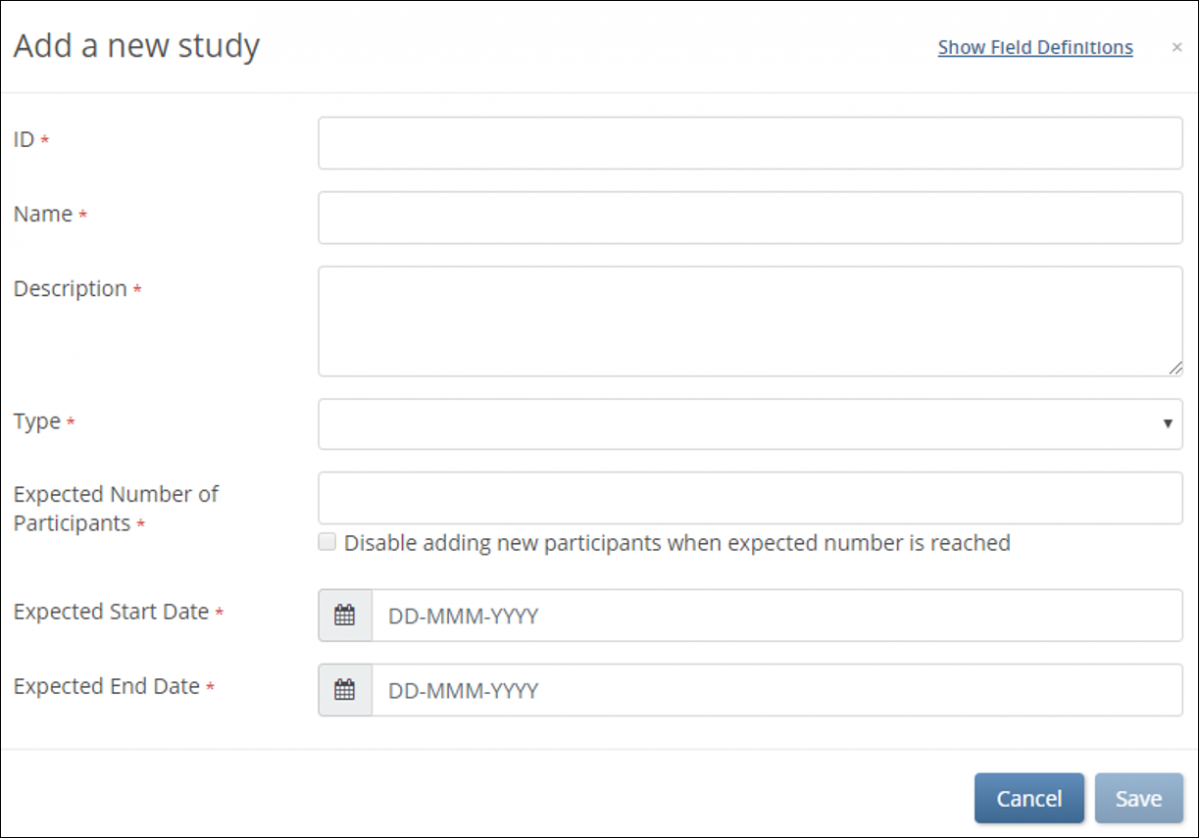
4. Click the Save button.
Note: To view definitions for a field, click the Show field definitions link, and hover your cursor over the field for which you want to see a definition.
To set a limit on the number of Participants that can be in the Study:
1. Enter a number in the Expected Number of Participants field.
2. Check the Disable adding new participants when expected number is reached checkbox.
For example, if you enter 100 in the Expected Number of Participants field, once the number of Participants reaches 100, you can no longer add Participants.
Note: The total number of Participants includes Participants with a Status of Available or signed. Participants with a status of removed are not included in the total number. You can view the current number or percentage of Participants in each Status in the Participant Status Count table on the Home screen of your study.
To change the Study Settings:
1. Click the Gear button and select Settings from the drop-down list in one of the following locations:
– The My Studies screen
– The Design screen
– The Share screen
2. To open the Study Properties screen, click the Edit link to the right of Study Settings.
3. Change data in the appropriate field(s).
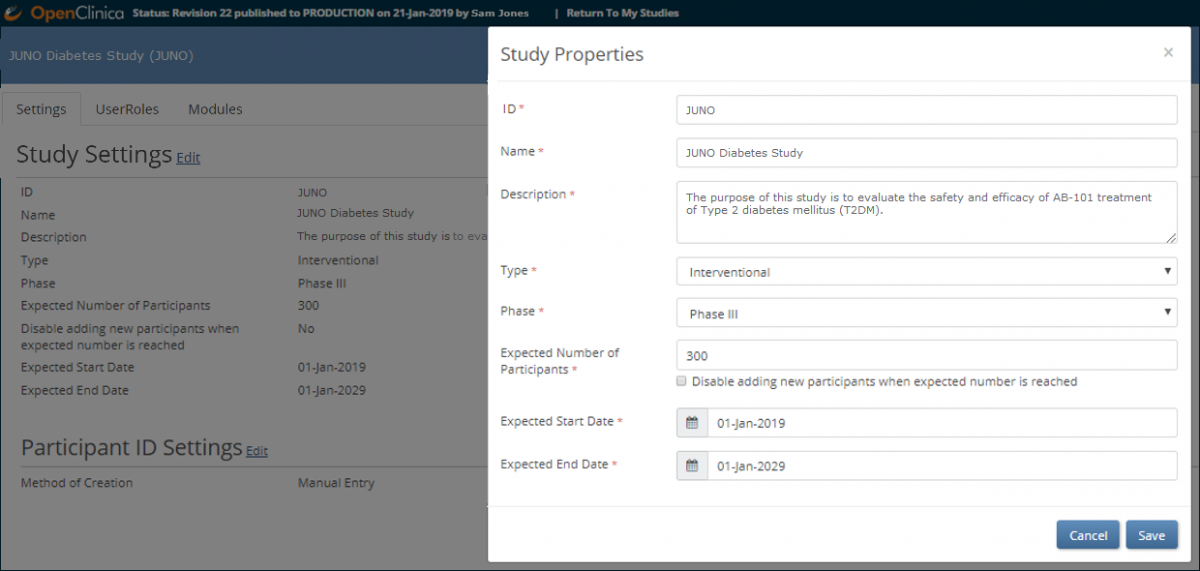
Note: You can change settings at any time. When you change data in the Settings window, you do not need to republish the study, and changes take effect immediately. Click the Save button.
