After a Visit including an eConsent form has been scheduled and the participant has been invited to the study, the participant can electronically sign the eConsent form.
Sign an eConsent form (Participants):
When a participant opens their dashboard, they will see the eConsent form on their dashboard with the date that the Visit event was scheduled.
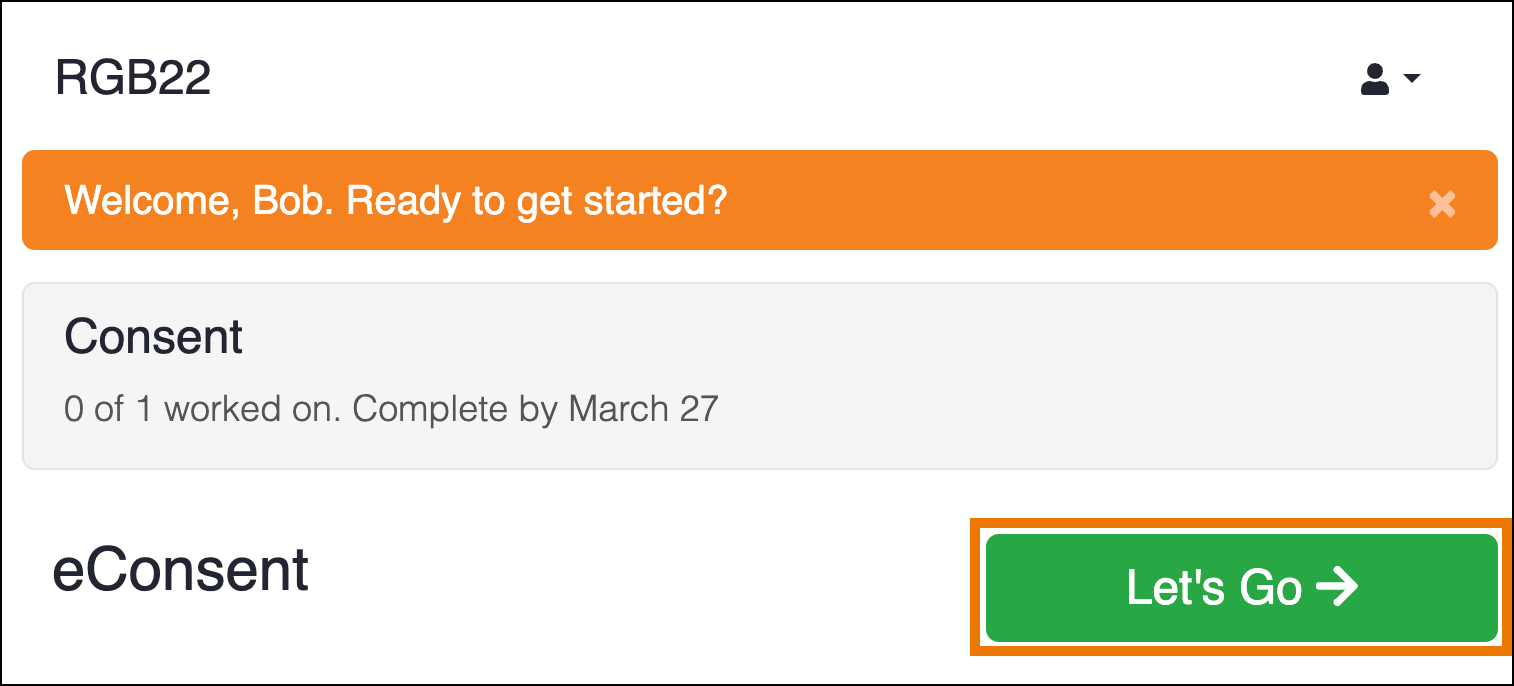
After the participant clicks Let’s Go, they will see the form to fill out. For the eConsent form, in addition to any other text or input items in the form definition, the Participant will see the consent checkbox to indicate they are ready to add their formal signature. The text on the form will vary based on your study configuration. In the image below, the question asking whether the Participant is willing to participate in the study is an example of a question configured during study design.

Once the participant has answered the study-specific questions, they can click the checkbox to confirm their agreement to participate in the study. To proceed, they must enter:
- Participant ID: This will be displayed on the screen.
- Access Code: This is provided in the email they received alerting them that the eConsent visit was available for signature.
If they don’t remember or have lost their access code, they can request a new one by clicking “click here”.

The new access code will be sent to their email address, mobile phone number, or both (if both are available).

After the participant enters their Participant ID and access code, they will click Sign. The Sign button becomes clickable only after both the Participant ID and access code have been entered.

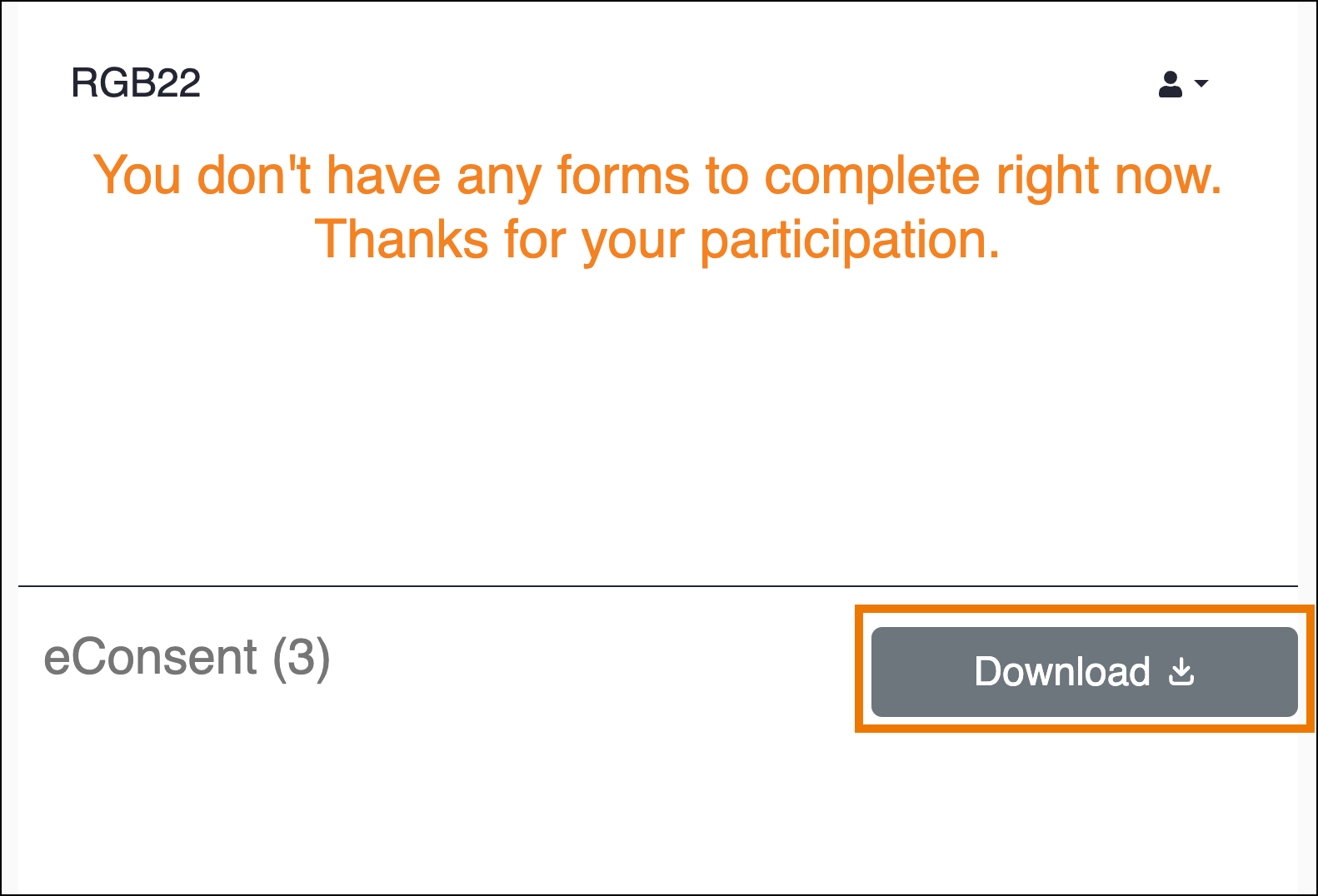
With the eConsent signed, the form will automatically be marked complete. When the participant returns to their dashboard, they will notice the eConsent form will appear at the bottom with a grey download button which allows them to download a copy of their eConsent form. This download option will remain available to the Participant if they return to the dashboard in the future.