Overview
The features of OC4 data entry include the following:
- Participant Matrix: Easily see the data entry status of participants.
- Auto-save: Automatically save during data entry.
- Conditional field display: Only see relevant fields during data entry.
- Automatic calculations and data checks: Automatically use calculations and checks.
- Queries: Easily create or view queries.
Data Entry Features
Concurrency Locking:
Two users cannot access the same Form for data entry/editing at the same time. If another user is currently editing the Form or working with queries on the Form you selected, a warning appears, indicating that the Form is in use by another user:
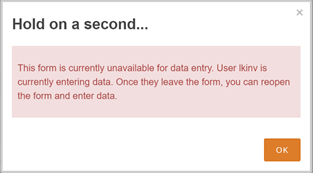
You can still view the data on that Form, but you cannot edit data or add/modify queries associated with that Form. When the user listed in the message closes the Form, logs out, or times out of OpenClinica, you can enter and edit data again.
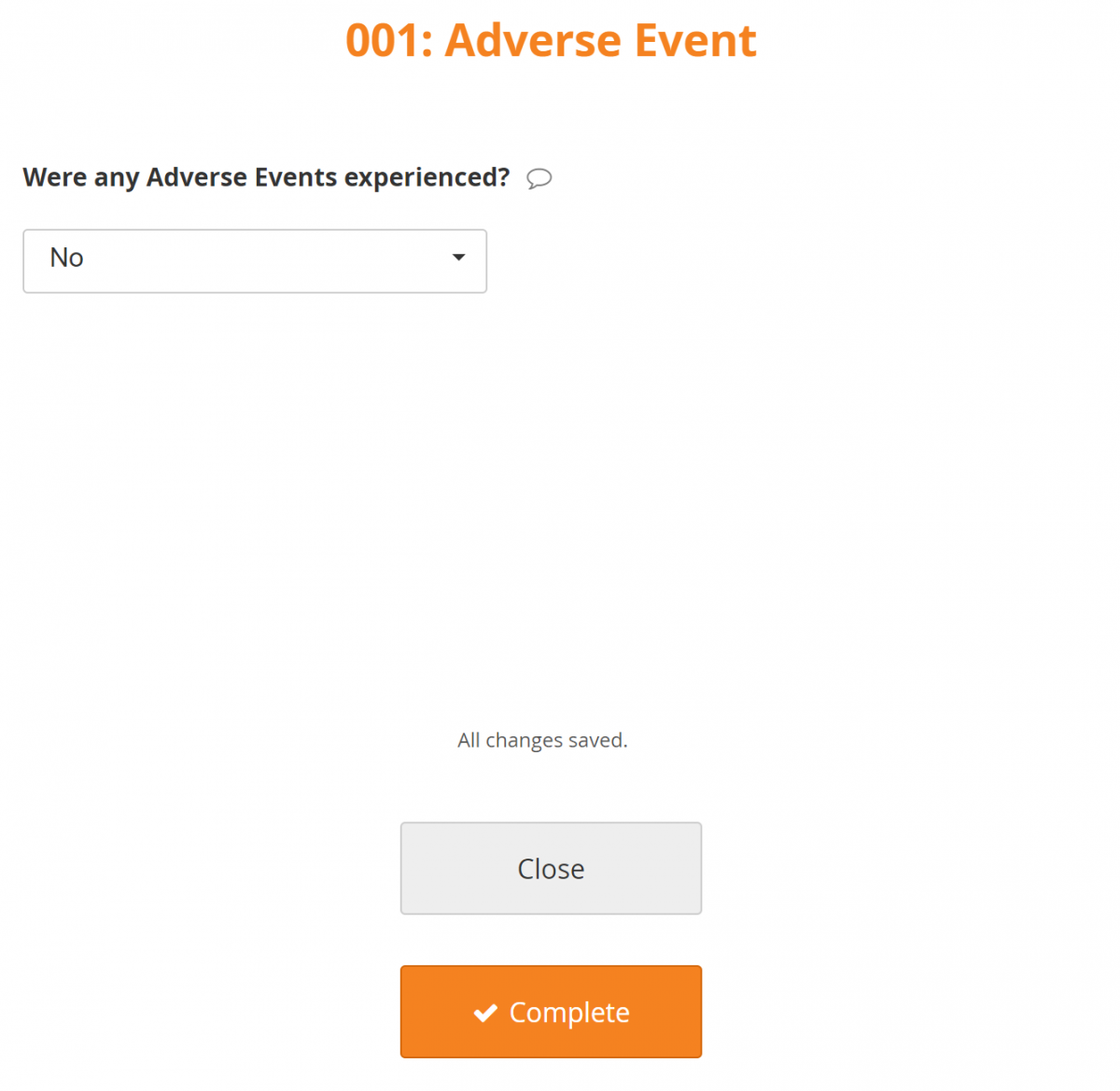
Conditional Field Display:
Only fields that are relevant based on values that you enter in other fields appear.
For example, detailed questions about the Adverse Event are only available if the response to Were any Adverse Events experienced? is Yes.
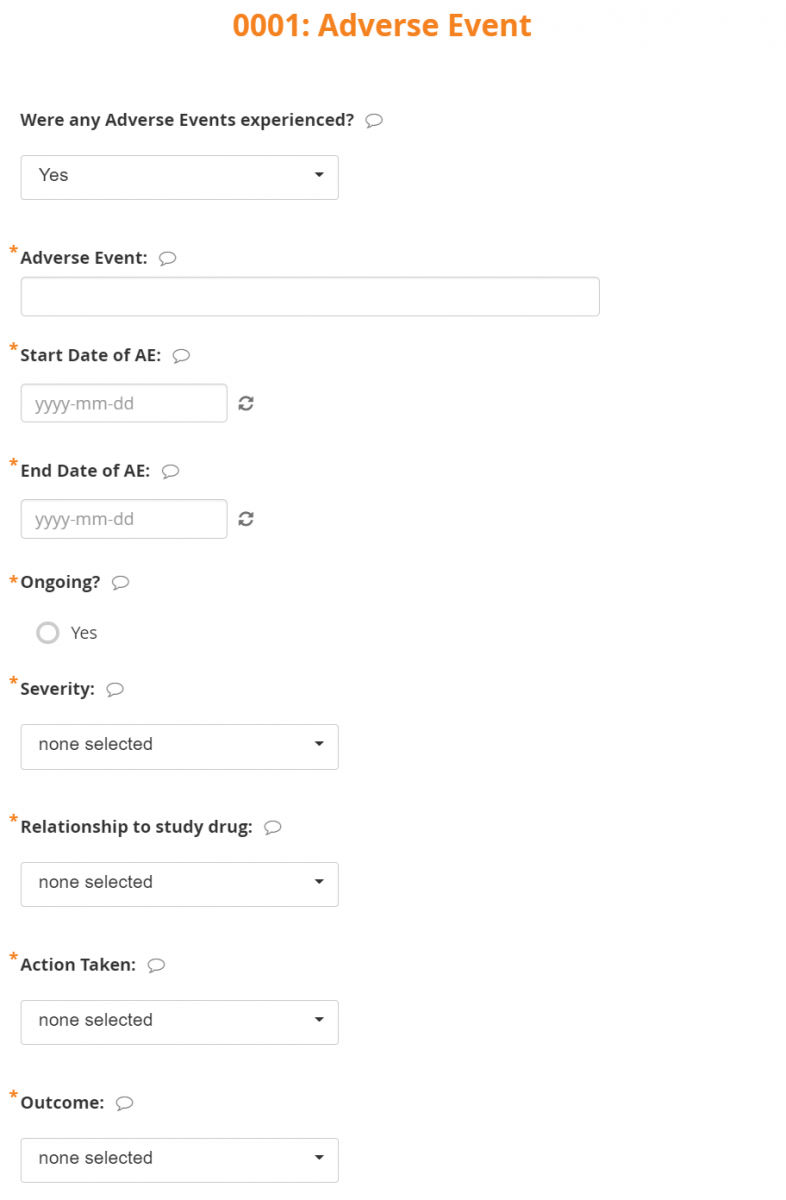
If the answer is No, the additional questions are not displayed.
Edit Checks:
When you click Close or Complete, the system evaluates the following:
- If you have responded to all required fields
- If you have entered invalid information (For example, if there is a constraint specifying that your response to the Oral Temperature cannot exceed 110 and you enter 150)
- If a value from prior data entry exists in a field that was removed.
Edit checks can be hard or soft.
Hard Edit Checks: Prevent you from navigating within the form or completing it. You can still close it.
Soft Edit Checks: Allow you to navigate within the form and close it, but prevent you from completing the Form.
The system will display a message, depending on the type of edit check. If you cannot resolve the error, the system suggests that you add a query.
Edit, Read-Only, and Review-Only Mode
Edit Mode: Allows data entry and adding or editing queries; Default for Data Managers, Investigators, Data Entry Users, and Data Specialists (Monitors cannot access Forms in this mode.)
Review-Only Mode: Allows adding or editing queries but user cannot enter data. Monitors automatically access Forms in this mode.
Read-Only Mode: View only (Does not allow data entry or adding/editing queries); User accesses Form in this mode automatically if the Event/Form is locked or another user is entering data.
Before You Enter Data
To enter data into OpenClinica, you must do the following:
- Add a Participant
- Schedule an Event
See Add Participants for more information on adding Participants.
Scheduling an Event
Once a Participant has been added, you can schedule events and start to enter data.
You can schedule events from the Participant Matrix, Participant Details screen, or the Tasks menu.
To Schedule a Visit-Based Event from the Participant Matrix:
- Click on the Schedule button for the Participant and the Event that you want to schedule.
- Select Schedule.
- Select a Study Event Definition from the drop-down list.
- (Optional) Select a Start Date/Time. The current date is the start date by default, but you can change it.
- (Optional) Select an End Date/Time.
- (Optional) To schedule additional Events, click Schedule Another Event, and enter information for that Event. Repeat as needed.
- To go to the Participant Details screen to enter data, click the Proceed to Enter Data button.
To Schedule a Visit-Based Event from the Participant Details screen:
- Click the Participant ID or View button next to a Participant.
- Under the Visits header on the Participant Details screen, click Add New.
- Select a Study Event Definition from the drop-down list.
- (Optional) Select a Start Date/Time. The current date is the start date by default, but you can change it.
- (Optional) Select an End Date/Time.
- (Optional) To schedule additional Events, click Schedule Another Event, and enter information for that Event. Repeat as needed.
- To go to the Participant Details screen to enter data, click the Proceed to Enter Data button.
To Schedule a Visit-Based Event from the Tasks menu:
- Click the Tasks button in the header bar of Runtime.
- Select Schedule Event.
- Enter a Participant ID in the Participant ID field.
- Select a Study Event Definition from the drop-down list.
- (Optional) Select a Start Date/Time. The current date is the start date by default, but you can change it. (Optional) Select an End Date/Time.
- (Optional) To schedule additional Events, click Schedule Another Event, and enter information for that Event. Repeat as needed.
- To go to the Participant Details screen to enter data, click the Proceed to Enter Data button.
To Schedule a Common Event from the Participant Details screen:
- Click the Participant ID or View button next to a Participant.
- Under a header for a common event on the Participant Details screen, click Add New.
Note: For Common Events, such as Adverse Events and Concomitant Medications, a visit date is not required, and the Add New button opens the form directly.
Entering Data
To enter data directly into Forms:
- Click the Add New button to add a Form associated with the Event.
- Click the Enter/Edit button in the Actions column to open the Form.
- Enter information into each field.
To:
- Continue to the next page of the Form, click the Next button.
- Mark data entry complete, click the Complete button.
- Close the Form and continue data entry later, click the Close button.
Note: If you cannot access a Form, the access to that Form might be restricted in your Study.
To download data for files, audio recordings, videos, images, annotations, drawings, or signatures (which are not 21 CFR Part 11 compliant), click the Download button that appears in the field. This button is available in Edit, Review-Only, and Read-Only modes.
Event/Form Independent Attributes:
Event/Form Attributes are separate from the Event/Form Statuses. Each Event or Form has a single status, but can have multiple attributes. For example, an Event might be Completed, Signed, and Locked.
The Participant Matrix
Once you have entered data for Participants, use the Participant Matrix to view and navigate Participant records.
The Participant Matrix lists visit-based events across the top and participant IDs down the side. Each icon represents the status of the participant/event combination. A legend of the icons is listed on the left side of the screen. Hover over the icons in the Participant Matrix to see more details about the participant event. Click an icon for options to view and/or edit data, depending on your access.
You can filter Events by status, including the independent statuses of Locked and Signed. You can also filter Events with Not Locked and Not Signed.
Note: If you filter Events with a status of Not Started, the Participant Matrix only includes Events that were previously started and then deleted, i.e. Events that have never been started are excluded. If you have a repeating Event for a Participant, the Participant Matrix shows the least complete status. For example, if the first event is data entry strated and the second event is completed, only the status of data entry started will display.
For information on removing, reassigning, and restoring Participants, see Review and Manage Data.
The Participant Details screen
To access the Participant Details screen, click on the Participant ID or the View button that corresponds to that Participant.
The Participant Details screen is divided into sections. Under General Information, there are sections for Visit-Based Events, Common Repeating Events, and Common Non-Repeating Events.
The General Information section displays information about the Participant, the Study, the Site, and the Participant Status.
Participant IDs:
- If the study has been set up for manual entry of Participant IDs, the Edit link displays above the Participant information, and the Participant ID can be edited.
- If the study has been set up for system-generated Participant IDs, the Edit link is not available.
- If the system-generated Participant ID is incorrect and must be edited, a Data Manager can:
- Go to the My Studies screen, and click the Settings button on the study card.
- Click the Edit link next to Participant ID Settings.
- Change the setting to Manual.
- Go to the Participant Details screen.
- Click the Edit link under General Information.
- Change the Participant ID.
- Click the Update button.
Common events collect data that is not necessarily related to a specific visit, but may occur between visits (e.g., adverse events, concomitant medications, or early termination). When you view the participant detail record, all visit-based events are listed first, followed by the common events.
The Participant Details page includes Visit-based events and related forms followed by any common events and related forms.
The page initially opens with the visit-based events displayed and all common events collapsed. To expand the common events sections, click the Expand All link to expand all common events, or click an individual common event section to expand only that section.
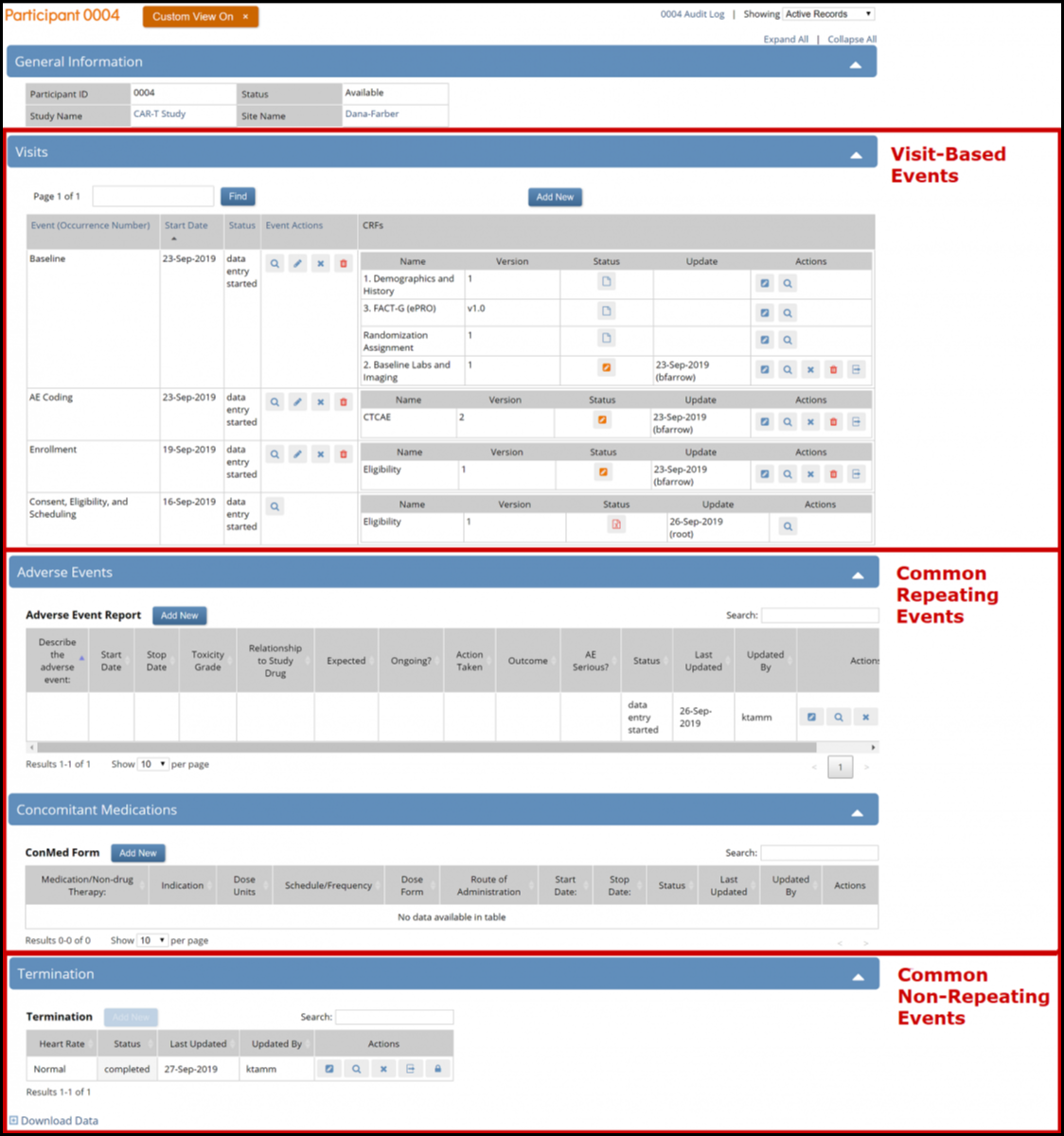
The Update Event Screen
To update an Event status, click the Edit button in the Event Actions column of the Participant Details screen.
Available statuses are Data Entry Started, Completed, Stopped, and Skipped.
If you are a Data Manager or Administrator, you can click the Lock Event button at the bottom of the screen to lock the Event.
If you are an Investigator or Data Specialist, you can click the Sign Event button to sign. You cannot remove an Event from this screen.
The Enter/Validate Data Screen
This screen displays details of the Event and Forms. You can access this screen by clicking the View button next to the Event on the Participant Details screen.
Event and Form Statuses/Actions
Events appear on the Participant Matrix, Participant Details screen, and many other screens throughout Runtime. If you click on the Event on the Participant Matrix, you can choose from some of the actions.
Note: If you filter Events with a status of Not Started, the Participant Matrix only includes Events that were previously started and then deleted, i.e. Events that have never been started are excluded.
Form Statuses and Attributes do not appear on the Participant Matrix, but do appear on the Participant Details screen, and many other screens throughout Runtime.
On the Participant Details screen, for Visit-based Events, the Attributes appear under the status in the Status column for Events and to the right of the status icon in the Status column for Forms. Actions are available on both sides of the screen for Events and Forms.
On the Participant Details screen, for Common Events, the Attributes appear to the right of the status in the Status column. Actions are available in the last column in the table
Below are tables that display the statuses and actions you can take on Events and Forms. Below the tables are instructions on how to perform each action.
Event Statuses:
Icon | Status | Description |
 | Not Scheduled | The Event has not been scheduled. Common Events, such as Adverse Events remain in this status throughout the study. |
| Scheduled | The Event has been scheduled, but no data has been entered. |
 | Data Entry Started | A user has started to enter data, but not all of the Forms in the Event have a status of completed. |
| Completed | A user has completed data entry for at least one Form in the Event. If further changes are needed in that Form, you are required to provide a reason for change. |
| Skipped | The user has decided not to complete the Event. Any data that has been entered can still be viewed and/or exported. You can select this setting from the dropdown menu on the Update Event screen when the current status is scheduled. |
| Stopped | The Participant has temporarily stopped participating in the study. You can select this status from the dropdown menu on the Update Event screen when the current status is data entry started. |
| Removed | The Event has been removed. Users can still view Forms. This will supersede any of the other statuses. |
Event Attributes:
Icon | Status | Description |
| Signed | The Event has been signed. This icon appears in addition to the status.
Note: If an Event is signed, changes to an item in a Form in that Event removes the signature. This also occurs if an Event status is changed from completed, stopped, skipped or Not Scheduled after being signed. In addition, this changes the Participant Status from signed to available and the Event Status to completed. Archiving/unarchiving or removing a Form will unsign the Event. The exception is that when archiving/unarchiving, a Form with a status of Not Started will not be unsigned.
Multiple users can sign an Event, so even if an Event has already been signed, the Sign Action will still be available. If there are multiple signatures, the most recent one appears on the Form, and the others appear in the Audit Log. |
| Archived | The Event was archived in Study Designer. This icon appears in addition to the status. |
| Locked | A Data Manager locked the Event. No data can be added, and the Event cannot be removed. This icon appears in addition to the status. |
Event Actions:
Icon | Action | Description |
| View | View the Event details on the Enter/Validate Data screen. |
| Edit | Edit the scheduled date/time. |
| Lock | Prevent other users from editing or removing Forms. Users can still view and sign Forms. |
| Unlock | Allow other users to edit or remove Forms that were previously locked. |
| Remove | Remove the Event from the study. This is not permanent. |
| Restore | Restore an Event that was removed from the study. All data is restored. |
| Sign | Approve the data for each Form in the Event. All Forms in the Event must either be completed or not scheduled.
Note: If an Event is signed, changes to an item in a Form in that Event removes the signature. This also occurs if an Event status is changed from completed, stopped, skipped or Not Scheduled after being signed. In addition, this changes the Participant Status from signed to available and the Event Status to completed. Archiving/unarchiving or removing a Form will unsign the Event. The exception is that when archiving/unarchiving, a Form with a status of Not Started will not be unsigned.
Multiple users can sign an Event, so even if an Event has already been signed, the Sign Action will still be available. If there are multiple signatures, the most recent one appears on the Form, and the others appear in the Audit Log. |
Form Statuses:
Icon | Status | Description |
| Not started | The Event has been scheduled, but no data has been entered on the Form. |
| Initial Data entry | The user has opened the Form and/or started to enter data, but the Form has not been completed. |
| Completed | The user has completed data entry on this Form. |
| Removed | This Form has been removed or the Event it is in has been removed. Users can still view the Form. This will supersede any of the other statuses. |
Form Attributes:
Icon | Status | Description |
| Signed | The Event has been signed. This icon appears in addition to the status.
Note: If an Event is signed, changes to an item in a Form in that Event removes the signature. This also occurs if an Event status is changed from completed, stopped, skipped or Not Scheduled after being signed. In addition, this changes the Participant Status from signed to available and the Event Status to completed. Archiving/unarchiving or removing a Form will unsign the Event. The exception is that when archiving/unarchiving, a Form with a status of Not Started will not be unsigned.
Multiple users can sign an Event, so even if an Event has already been signed, the Sign Action will still be available. If there are multiple signatures, the most recent one appears on the Form, and the others appear in the Audit Log. |
| Archived | The Event was archived in Study Designer. This icon appears in addition to the status. |
| Locked | A Data Manager locked the Event. No data can be added, and the Event cannot be removed. This icon appears in addition to the status. |
Form Actions:
Icon | Action | Description |
| Enter/Edit | Enter or edit data in the Form. |
| View | View data in the Form. |
| Remove | Remove the Form from the Event. This is not permanent. |
| Restore | Restore a Form that was previously removed. All data is restored. |
| Clear Form | Clear all data in the Form. This resets the Form to Not Started and closes all associated queries, but audit history is retained. |
| Reassign a CRF to a new Version | Change the version of the Form if data has already been entered. |
Event Actions
To View an Event:
Click the View button in the Event Actions column on the Participant Details screen.
Edit an Event:
Click the View button in the Event Actions column on the Participant Details screen.
To Lock an Event:
If you are a Data Manager you can lock an Event so that no Form data can be entered or edited and Forms cannot be removed, restored, or cleared.
Click the Lock button in the Event Actions column on the Participant Details screen.
or
Click the Edit button in the Event Actions column on the Participant Details screen. On the Update Study Event screen, click the Lock button under Other Actions.
To Sign an Event:
If you are an Investigator or Data Specialist, you can sign an Event to indicate that all Forms have been reviewed and approved.
If you are an Investigator or Data Specialist, you can sign an Event to indicate that all Forms have been reviewed and approved.
Events must have a status of completed, stopped, or skipped before they can be signed. The Event cannot have been archived or removed. To sign a participant event, first review the data captured in the event, then change the status to signed.
Click the Sign button in the Event Actions column on the Participant Details screen.
or
Click the Edit button in the Event Actions column on the Participant Details screen. On the Update Study Event screen, click the Sign button under Other Actions.
Note: If an Event is signed, changes to an item in a Form in that Event removes the signature. This also occurs if an Event status is changed from completed, stopped, skipped or Not Scheduled after being signed. In addition, this changes the Participant Status from signed to available and the Event Status to completed.
Archiving/unarchiving or removing a Form will unsign the Event. The exception is that when archiving/unarchiving, a Form with a status of Not Started will not be unsigned.
Multiple users can sign an Event, so even if an Event has already been signed, the Sign Action will still be available. If there are multiple signatures, the most recent one appears on the Form, and the others appear in the Audit Log.
To Remove an Event:
Click the Remove button in the Event Actions column on the Participant Details screen. This is replaced with the Restore button.
Note: If an Event is removed after being signed, the signature is invalidated, and if restored, the Form must be signed again.
To Restore an Event:
If you have removed an Event you want to restore, Click the Restore button in the Event Actions column on the Participant Details screen. This is replaced with the Remove button.
Forms
Note: If an Event is removed after being signed, the signature is invalidated, and if restored, the Form must be signed again.
To View a Form:
Click the View button in the Actions column on the Participant Details screen.
Enter/Edit Form Data:
Click the Enter/Edit button in the Actions column on the Participant Details screen. If you have permission to access the Form, you can start entering or editing data.
If you edit a Form that has already been completed, you must enter a reason for change.
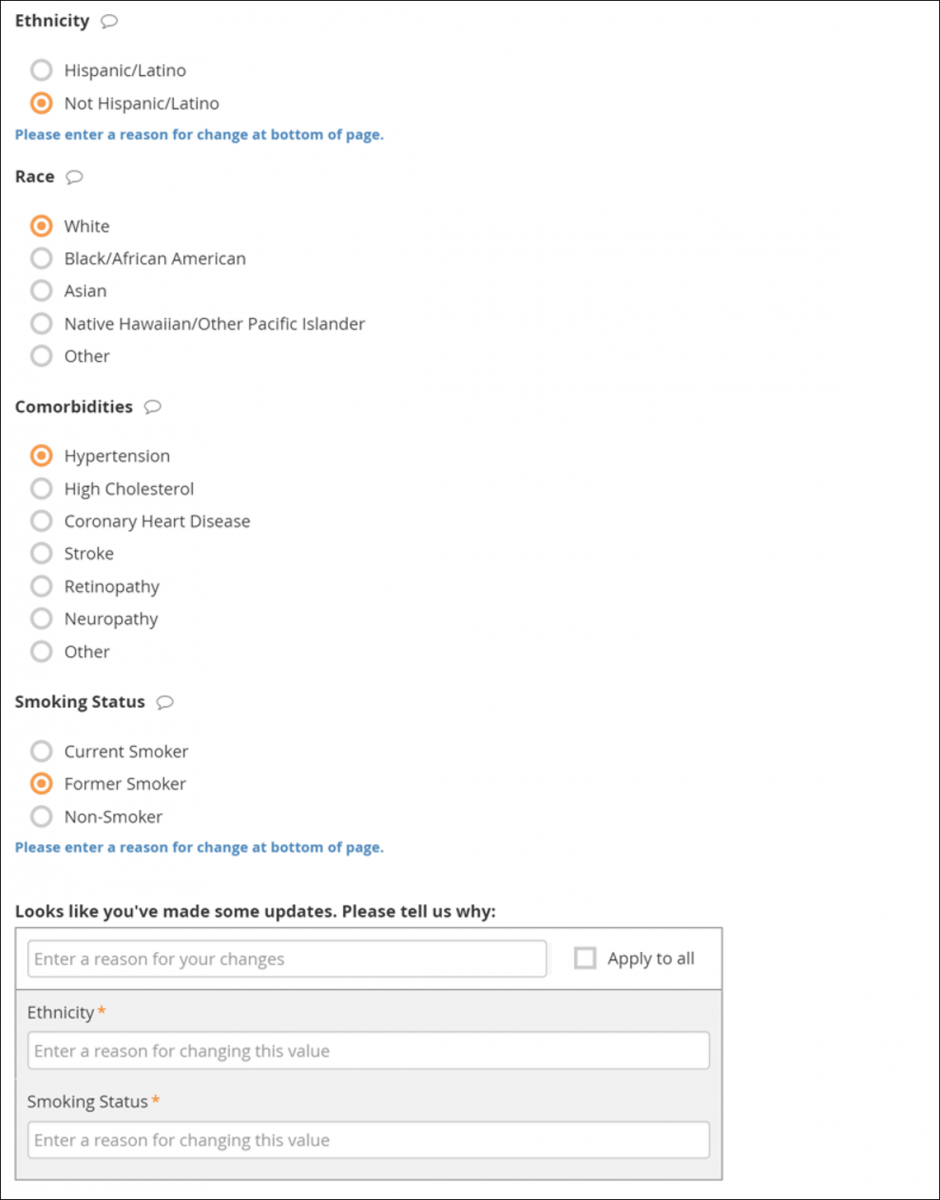
If your changes do not meet Form requirements, such as constraints, you will see a message alerting you that specific values have errors and must be changed.
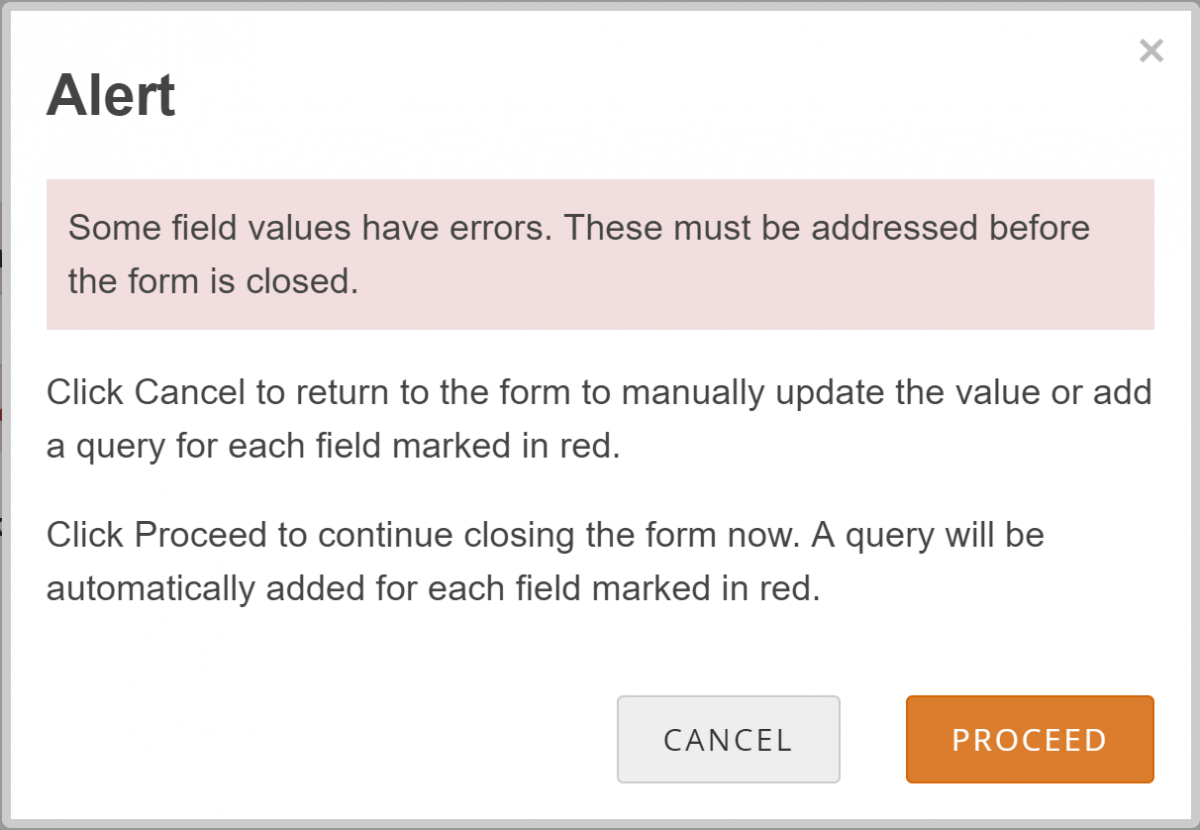
- Review the data and/or add a manual query:
- Click the Cancel button.
- Review the value to make sure you entered it correctly.
- If the value is correct, create the query that includes an appropriate message for that value. Otherwise, change the value.
- Allow the system to automatically create a query for the value in question
- Click the Proceed button.
- The system auto-generates a query based on the default message text defined in the Form.
To Remove a Form:
Click the Remove button in the Actions column on the Participant Details screen.
Note: If a Form is removed after being signed, the signature is invalidated, and if restored, the Form must be signed again.
To Restore a Form:
Click the Restore button in the Actions column on the Participant Details screen.
If a Form is removed after being signed, the signature is invalidated, and if restored, the Form must be signed again.
To Clear a Form:
To clear form data, click the Clear Form button in the Actions column on the Participant Details screen. The system does the following:
- Clears all data from the Form
- Closes all queries associated with the Form
- Removes a signature, if present
- Sets the Status of the Form to Not Started
- Records this action in the Participants Audit Log.
Printing a Form
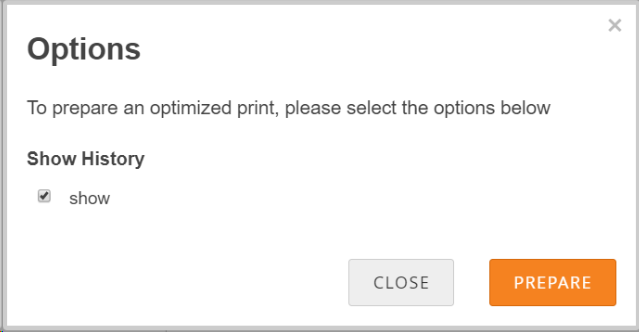
To Print a Form:
- Click the Print button that appears at the top of the screen for each Form.
- Enter information on the Options screen.
- (Optional) Check the checkbox next to Show to include Query and Edit History on the printed Form.
- Select a Paper Size.
- Select a Paper Orientation.
Note: The Paper Size and Paper Orientation fields appear only for Forms with the Style of theme-grid and printing options must be set correctly in your browser for these settings to take effect.
- Click the Prepare button to show a preview.
- Click the Print button.